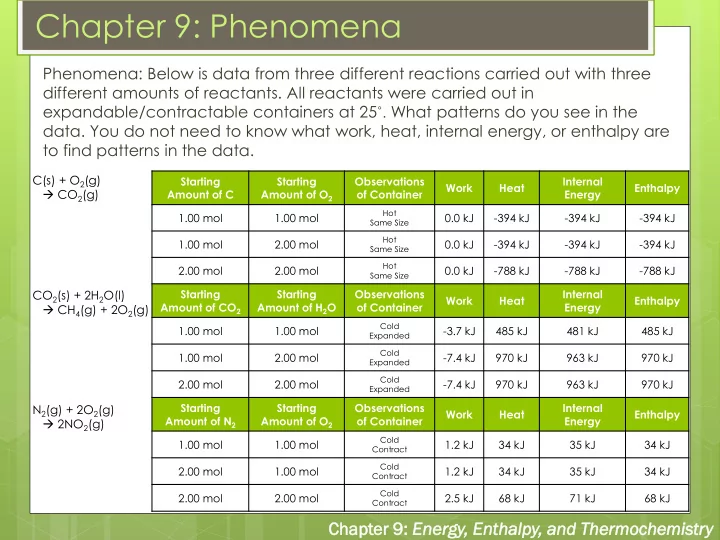

Chapter 9: Phenomena Phenomena: Below is data from three different reactions carried out with three different amounts of reactants. All reactants were carried out in expandable/contractable containers at 25 ˚. What patterns do you see in the data. You do not need to know what work, heat, internal energy, or enthalpy are to find patterns in the data. C(s) + O 2 (g) Starting Starting Observations Internal Work Heat Enthalpy CO 2 (g) Amount of C Amount of O 2 of Container Energy Hot 1.00 mol 1.00 mol 0.0 kJ -394 kJ -394 kJ -394 kJ Same Size Hot 1.00 mol 2.00 mol 0.0 kJ -394 kJ -394 kJ -394 kJ Same Size Hot 2.00 mol 2.00 mol 0.0 kJ -788 kJ -788 kJ -788 kJ Same Size CO 2 (s) + 2H 2 O(l) Starting Starting Observations Internal Work Heat Enthalpy Amount of CO 2 Amount of H 2 O of Container Energy CH 4 (g) + 2O 2 (g) Cold 1.00 mol 1.00 mol -3.7 kJ 485 kJ 481 kJ 485 kJ Expanded Cold 1.00 mol 2.00 mol -7.4 kJ 970 kJ 963 kJ 970 kJ Expanded Cold 2.00 mol 2.00 mol -7.4 kJ 970 kJ 963 kJ 970 kJ Expanded Starting Starting Observations Internal N 2 (g) + 2O 2 (g) Work Heat Enthalpy Amount of N 2 Amount of O 2 of Container Energy 2NO 2 (g) Cold 1.00 mol 1.00 mol 1.2 kJ 34 kJ 35 kJ 34 kJ Contract Cold 2.00 mol 1.00 mol 1.2 kJ 34 kJ 35 kJ 34 kJ Contract Cold 2.00 mol 2.00 mol 2.5 kJ 68 kJ 71 kJ 68 kJ Contract Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try
Chapter 9 Energy, Enthalpy, & Thermochemistry o Energy Facts Big Idea: Heat and work are o Internal Energy equivalent ways of o Enthalpy changing the energy o Hess’s Law of a system. The total o Calorimetry energy of an isolated o H 2 Fuel system is constant. The change in enthalpy of a reaction indicates whether a reaction is endothermic or exothermic. 2
Energy Facts Source of Percent Energy Petroleum 32% Coal 30% Natural Gas 24% Renewable 10% Natural Nuclear 4% Gas Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 3
Energy Facts 2014 Country Energy Country Energy Consumption Consumption/person (Quadrillion Btu) (MBtu) China 119.3 Qatar 1160 United Arad United States 98.0 800 Emirates Russia 30.7 Netherlands 750 India 24.3 Iceland 687 Japan 18.9 Kuwait 635 Canada 14.5 Singapore 633 Brazil 12.8 Bahrain 614 Germany 12.7 Canada 427 Korea, South 11.1 Norway 407 Iran 10.7 Saudi Arabia 398 United 314 States (#14) http://www.eia.gov/cfapps/ipdbproject/IEDIndex3.cfm?tid=44&pid=44&aid=2 Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 4
US Energy US Sources of Energy 2014 (Quadrillion Btu) Source % % % of World US US Energy 2014 2014 2009 Petroleum 32% 35% 37% Natural 24% 28% 25% Gas Coal 30% 18% 21% Renewable 10% 10% 8% Nuclear 4% 8% 9% http://www.eia.doe.gov/aer/pecss_diagram.html Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 5
Energy Facts How do we generate electricity? Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 6
Internal Energy System: The object of study. Surrounding: The region outside the system. Internal Energy ( E ): The capacity to do work or to produce heat. Temperature ( T ): How hot or cold an object is. Heat ( q ): The energy that is transferred as a result of a temperature difference between a system and its surroundings. Not ote: If heat enters the system q is positive (endothermic reaction). If heat leaves the system q is negative (exothermic reaction). Work ( w ): The energy expended during the act of moving an object against an opposing force. Not ote: If the system expands, w is negative. If the system contracts, w is positive. Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 7
Internal Energy Student Question Which of these changes results in an increase in the internal energy of the system? a) The system absorbs heat and does work on the surroundings. b) The system releases heat and does work on the surroundings. c) The system absorbs heat and has work done on it by the surroundings. d) The system releases heat and has work done on it by the surroundings. Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 8
Internal Energy Student Question Helium gas, at a pressure of 2 Pa, is placed in a container with a movable piston. On the other side of the piston is a vacuum. The He gas is allowed to expand such that the volume of the helium goes from 2 m 3 to 4 m 3 . How much work does the helium gas do? a) 8 J b) 4J c) 0 J d) -4 J e) None of the above Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 9
Internal Energy Heat Capacity (C): The ratio of heat supplied to the temperature rise produced (units 𝐾 ℃ ) Molar Heat Capacity: The heat capacity per mole of substance (units 𝑛𝑝𝑚∙℃ ) 𝐾 Not ote: Many books use C m for molar heat capacity. Specific Heat Capacity: The heat capacity per gram of substance (units J g∙℃ ) Not ote: Many books use C S for specific heat capacity. Not ote: If the subscript V is added to any of the heat capacities it is the heat capacity at constant volume. If the subscript P is added to any of the heat capacities it is the heat capacity at constant pressure. Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 10
Internal Energy Molar Heat Capacities at 298K Specific Heat Capacities C C V C P C P -C V Gas Substances 𝑲 𝑲 𝑲 𝑲 𝒉∙𝑳 𝒏𝒑𝒎∙𝑳 𝒏𝒑𝒎∙𝑳 𝒏𝒑𝒎∙𝑳 He, Ne, Ar 12.47 20.80 8.33 H 2 O(l) 4.18 H 2 20.54 28.86 8.32 H 2 O(s) 2.03 N 2 20.71 29.03 8.32 Al(s) 0.89 N 2 O 30.38 38.70 8.32 Fe(s) 0.45 CO 2 28.95 37.27 8.32 Hg(l) 0.14 C 2 H 6 44.60 52.92 8.32 C(s) 0.71 q nC T q mC T Not ote: The smaller the heat capacity, the faster the transfer of heat. Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 11
Internal Energy C V (Monatomic Ideal Gas) Know Putting it together Δ E=q+w= Δ PE+ Δ KE Potential Energy Kinetic Energy Work Only true for ideal monatomic gases Heat Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 12
Internal Energy C P (Monatomic Ideal Gas) Know Putting it together Δ E=q+w= Δ PE+ Δ KE Potential Energy Kinetic Energy Work Only true for ideal monatomic gases Heat Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 13
Internal Energy State Function: A property of a substance that is independent of how a substance was prepared. Are Δ E, q, and w state functions? 1 mol of monatomic ideal gas Path A 1.0 atm, 1.0 L 1.0 atm, 2.0 L 2.0 atm, 2.0 L Path B 1.0 atm, 1.0 L 2.0 atm, 1.0 L 2.0 atm, 2.0 L Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 14
Internal Energy Path B (Monatomic Ideal Gas) 1.0 atm, 1.0 L 2.0 atm, 1.0 L 2.0 atm, 2.0 L Step 1 (Constant Volume) Heat Work 𝑟 = 𝑜𝐷 𝑊 ∆𝑈 𝑥 = −𝑄 𝑓𝑦 ∆𝑊 = 0.0 𝐾 𝐷 𝑊 = 3 2 𝑆 ( Δ V=0) (For monatomic ideal gas) Internal Energy 𝑟 = 𝑜 3 2 𝑆∆𝑈 ∆𝐹 = 𝑟 + 𝑥 Problem: We do not know T ∆𝐹 = 150 𝐾 + 0.0 𝐾 ∆𝑄 𝑊 = 𝑜𝑆∆𝑈 ∆𝐹 = 150 𝐾 𝑟 = 3 2 ∆𝑄 𝑊 = 3 2 𝑄 𝑔 − 𝑄 𝑗 𝑊 𝑟 = 3 2 2.0 𝑏𝑢𝑛 − 1.0 𝑏𝑢𝑛 1.0 𝑀 𝑟 = 1.5 𝑀 ∙ 𝑏𝑢𝑛 = 150 𝐾 Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 15
Internal Energy Path B (Monatomic Ideal Gas) 1.0 atm, 1.0 L 2.0 atm, 1.0 L 2.0 atm, 2.0 L Step 2 (Constant Pressure) Heat Work 𝑟 = 𝑜𝐷 𝑄 ∆𝑈 𝑥 = −𝑄 𝑓𝑦 ∆𝑊 𝐷 𝑄 = 5 2 𝑆 w = −𝑄 𝑓𝑦 𝑊 𝑔 − 𝑊 (For monatomic ideal gas) 𝑗 w = − 2.0 𝑏𝑢𝑛 2.0 𝑀 − 1.0 𝑀 𝑟 = 𝑜 5 2 𝑆∆𝑈 𝑥 = −2.0 𝑀 ∙ 𝑏𝑢𝑛 = −2.0 × 10 2 𝐾 Problem: We do not know T Internal Energy 𝑄∆𝑊 = 𝑜𝑆∆𝑈 𝑟 = 5 2 𝑄∆𝑊 = 5 2 𝑄 𝑊 𝑔 − 𝑊 ∆𝐹 = 𝑟 + 𝑥 𝑗 ∆𝐹 = 5.0 × 10 2 𝐾 + −2.0 × 10 2 𝐾 𝑟 = 5 2 2.0 𝑏𝑢𝑛 2.0 𝑀 − 1.0 𝑀 ∆𝐹 = 3.0 × 10 2 𝐾 𝑟 = 5.0 𝑀 ∙ 𝑏𝑢𝑛 𝑟 = 5.0 × 10 2 𝐾 Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 16
Internal Energy Path A 1.0 atm, 1.0 L 1.0 atm, 2.0 L 2.0 atm, 2.0 L Path B 1.0 atm, 1.0 L 2.0 atm, 1.0 L 2.0 atm, 2.0 L Path B (totals) Path A (totals) 𝑟 = 150 𝐾 + 5.0 × 10 2 𝐾 q = 550 J q = 650 𝐾 𝑥 = 0.0 𝐾 + −2.0 × 10 2 𝐾 𝑥 = −1.0 × 10 2 𝐾 w = −2.0 × 10 2 𝐾 ∆𝐹 = 150 𝐾 + 3.0 × 10 2 𝐾 ∆𝐹 = 450 𝐾 ∆𝐹 = 450 𝐾 Chapt pter er 9: Energy, Enthal halpy, and d Therm ermoc ochem emis istr try 17
Recommend
More recommend