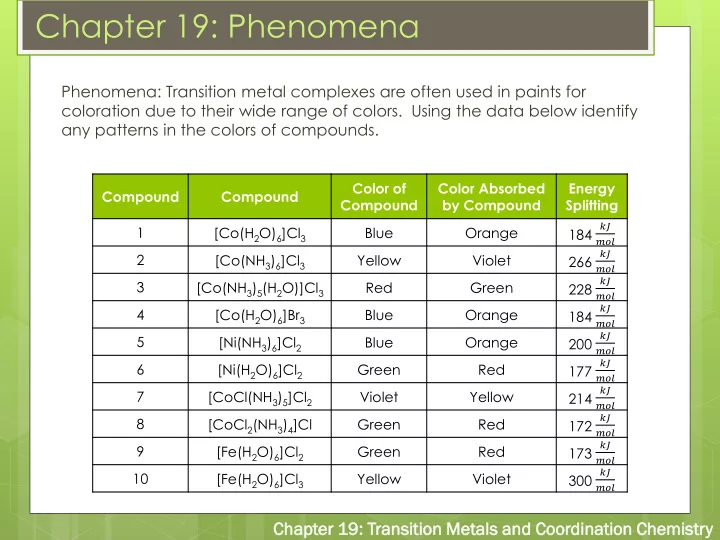

Chapter 19: Phenomena Phenomena: Transition metal complexes are often used in paints for coloration due to their wide range of colors. Using the data below identify any patterns in the colors of compounds. Color of Color Absorbed Energy Compound Compound Compound by Compound Splitting 𝑙𝐾 1 [Co(H 2 O) 6 ]Cl 3 Blue Orange 184 𝑛𝑝𝑚 𝑙𝐾 2 [Co(NH 3 ) 6 ]Cl 3 Yellow Violet 266 𝑛𝑝𝑚 𝑙𝐾 3 [Co(NH 3 ) 5 (H 2 O)]Cl 3 Red Green 228 𝑛𝑝𝑚 𝑙𝐾 4 [Co(H 2 O) 6 ]Br 3 Blue Orange 184 𝑛𝑝𝑚 𝑙𝐾 5 [Ni(NH 3 ) 6 ]Cl 2 Blue Orange 200 𝑛𝑝𝑚 𝑙𝐾 6 [Ni(H 2 O) 6 ]Cl 2 Green Red 177 𝑛𝑝𝑚 𝑙𝐾 7 [CoCl(NH 3 ) 5 ]Cl 2 Violet Yellow 214 𝑛𝑝𝑚 𝑙𝐾 8 [CoCl 2 (NH 3 ) 4 ]Cl Green Red 172 𝑛𝑝𝑚 𝑙𝐾 9 [Fe(H 2 O) 6 ]Cl 2 Green Red 173 𝑛𝑝𝑚 𝑙𝐾 10 [Fe(H 2 O) 6 ]Cl 3 Yellow Violet 300 𝑛𝑝𝑚 Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry
Chapter 19 Transition Metals & Coordination Chemistry Big Idea: The properties of d - block metals are o The d -Block Elements and governed by the Their Compounds availability of d -orbitals o Coordination Compounds and their variable o Crystal Field Theory valence electrons. o Ligand Field Theory The physical properties of d -block complexes depends on the properties of the ligands bound to the metal. 2
d-Block Elements and Their Compounds Transition metals, are located in groups 3 through 11. They are called transition metals because they transition between the highly reactive s block metals and the much less reactive metals of group 12 and the p block. Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 3
d-Block Elements and Their Compounds Scandium Titanium Vanadium Chromium (Sc) (Ti) (V) (Cr) Facts • Reacts • Resistant to • Vanadium • Corrosion vigorously with corrosion compounds resistant water (protective come in a wide oxide skin) range of color due to its many • Requires strong oxidation states reducing agent for extraction from, its ores Uses • Few uses • Jet engines • Makes tough • Stainless steel steel for • Not essential to • Dental • Chrome automobile and life applications plating truck springs • Glazes for ceramics Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 4
d -Block Elements and Their Compounds Iron (Fe) Cobalt (Co) Nickel (Ni) Manganese (Mn) Facts • Not as corrosion • Most widely used • 70% of the resistant as d metal western world’s chromium but • Most abundant nickel comes more corrosion element on earth from ore that was resistant than iron brought close to the earth surface nearly 2 billion year ago by the violent impact of a huge meteor Uses • Alloying with steel • Main component • Alloying with steal • Used to make in steel (next stainless steal • Used to make biggest permanent • Nickel is alloyed component C up magnets found in with copper to to 2.1%) speakers make nickel • Essential to life coins • Essential to life Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 5
d -Block Elements and Their Compounds Copper (Cu) Zinc (Zn) Facts • Corrosion resistant • One of the coinage metals • High conductivity • Corrosion resistant Uses • Alloying: Brass (Cu • Used for + Zn) and Bronze galvanizing (Cu and Sn) • Used for electrical wires • Used for water pipes Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 6
d -Block Elements and Their Compounds The shape of the d- orbitals affect the properties of transition metals. The d -orbital lobes are far apart and so only weakly repel each other. The d -orbitals have low electron density near the nucleus therefore are not very effective at shielding. Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 7
d -Block Elements and Their Compounds Orange boxes are common oxidation numbers. Green boxes are other know states. Most d -block metals have more that one oxidation state other than 0. Elements close to the center of the row have the widest range of oxidation numbers. Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 8
d -Block Elements and Their Compounds Not ote: The ordering of the ns and (n-1)d energy level shifts once the (n-1)d orbitals contain e - causing the (n-1)d e - to be lower in energy than the ns electrons. Therefore, ns electrons are lost before (n-1)d electrons when forming transition metal ions. ns electrons are also lost before (n-2)f electrons. Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 9
d -Block Elements and Their Compounds Student Question What is the correct electron configuration for Ir 3+ ? a) [Xe]4f 13 5d 7 b) [Xe]6s 2 4f 14 5d 7 c) [Xe]4f 14 5d 6 d) [Xe]6s 2 4f 14 5d 4 e) None of the Above Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 10
Coordination Compounds Complex Ion: A charged species consisting of a metal ion surrounded by ligands. Ligand: A group attached to a central metal ion in a complex. Central grey # # # atom is # transition metal. Colored atoms ligands. Examples: [Fe(H 2 O) 6 ] 3+ [CoCl 4 ] 2- [NiBr 4 ] 2- [Ag(NH 3 ) 2 ] + Not ote: Complex ions are written inside brackets. If ligands are bound to a metal but the species formed is not an ion the system is still written in brackets ex: [Hg(CH 3 ) 2 ]. Not ote: Ligands are bond to the metal via coordinate covalent bonds (also know as dative bonds) these are covalent bonds in which one atom gives both of the electrons in the bond. Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 11
Coordination Compounds Formula Name H 2 O Aqua NH 3 Ammine Br - Bromo Chelate: A Cl - Chloro complex CN - Cyano CO Carbonyl containing at NH 2 CH 2 CH 2 NH 2 Ethylenediamine (en) least one OH - Hydroxo polydentate I - Iodo ligand that forms CH 3 NH 2 Methylamine - (N bonded to a ring of atoms. NO 2 Nitro metal) ONO - (O bonded to Nitrito metal) NO Nitrosyl Not ote: Bidentate ligands form C 2 O 4 2- Oxalato (ox) 2 bonds to the metal ion. SO 4 2- Sulfato SCN - Thiocyanto Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 12
Coordination Compounds Coordination Number: The number of bonds formed between the metal ion and the ligands in a complex ion. # # # # What is the Coordination Shape coordination Number number for: Octahedral 6 Tetrahedral 4 Square Planar 4 2 Linear Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 13
Coordination Compounds Student Question Determine the coordination number and oxidation number, respectively, of the transition metal ion[CrBr 2 (en) 2 ] + ? a) 4, 3 b) 4, 2 c) 6, 3 d) 6, 2 e) None of the Above Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 14
Coordination Compounds Coordination Compound: A compound composed of a complex ion and counter ion sufficient to give no net charge. Counter Ion: Anions or cations that balance the charge on the complex ion in a coordination complex. Exa xample: ple: K 3 [Fe(CN) 6 ] K + counter ion [Fe(CN) 6 ] 3- complex ion Not ote: Counter ions can dissociate in water (ionically bonded) while complex ions can not dissociate in water (covalently bonded). Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 15
Coordination Compounds Student Question How many of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO 3 ? [CrCl 3 (NH 3 ) 3 ] [Cr(NH 3 ) 6 ]Cl 3 [CrCl(NH 3 ) 5 ](OH) 2 Na 3 [Cr(CN) 6 ] a) 0 b) 1 c) 2 d) 3 e) 4 Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 16
Coordination Compounds Naming Coordination Complexes Cations (positively charged) are named before the 1. anions (negatively charged). Name the ligands first and then the metal atom or 2. ion. When writing the chemical formula of complex ions the metal comes first. Most neutral ligands have the same name as the 3. molecule. Exa xample: ple: NH 2 CH 2 CH 2 NH 2 (ethylenediamine) Exc xcept ptions ns: : H 2 O aqua NH 3 ammine CO carbonyl NO nitrosyl Anionic ligand (- charged) end in – o; 4. -ide (such as chloride) change to – o (chloro) -ate (such as sulfate) change to – ato (sulfato) -ite (such as nitrite) change to – ito (nitrito) Chapt pter er 19: Tra ransition sition Met etals s and Coor ordi dina nati tion on Chemi mistr stry 17
Recommend
More recommend