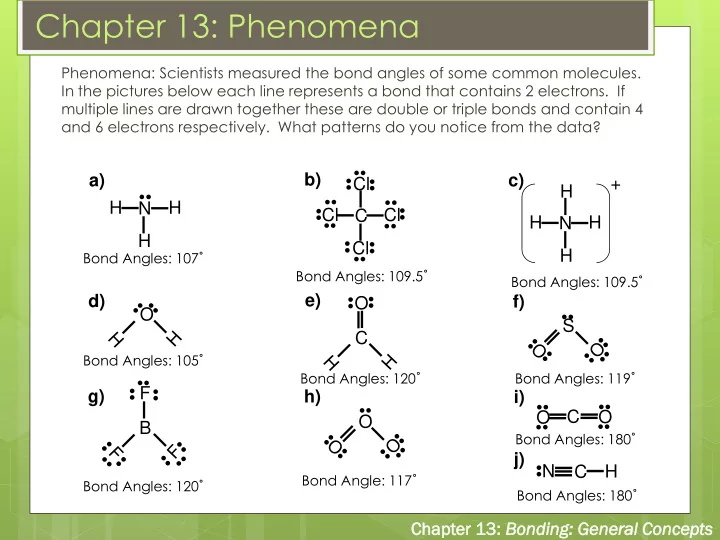

Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together these are double or triple bonds and contain 4 and 6 electrons respectively. What patterns do you notice from the data? b) a) c) + Cl H H H N Cl Cl C H H N H Cl H Bond Angles: 107 ˚ Bond Angles: 109.5 ˚ Bond Angles: 109.5 ˚ d) e) f) O O S C Bond Angles: 105 ˚ Bond Angles: 120 ˚ Bond Angles: 119 ˚ F g) h) i) C O O O B Bond Angles: 180 ˚ j) N C H Bond Angle: 117 ˚ Bond Angles: 120 ˚ Bond Angles: 180 ˚ Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts
Chapter 13 Bonding: General Concepts Big Idea: Bonds are formed from o Types of Bonding the attraction o Electronegativity between oppositely o Lewis Structures charged ions or by o Strength/Length of sharing electrons. Covalent Bonds Only the valence electrons participate o Shapes of Molecules in bonding. The shape (VSEPR) of the molecules o Polar Molecules maximize the distance between areas of high electron density. 2
Types of Bonding Ionic Bonds: Formed when a lower energy can be achieved by the complete transfer of one or more electrons from the atoms of one element to those of another; the compound is then held together by electrostatic attraction between the ions. Covalent Bonds: Formed when the lowest energy structure can be achieved by sharing electrons. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 3
Types of Bonding Ionic Bonds: Tend to be between a metal and a non metal. Metals: Usually lose their electrons. Nonmetals: Usually accept additional electrons. Not ote: In general, atoms gain or lose electrons until they have the same number of electrons as the nearest noble gas Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 4
Types of Bonding What ions do atoms form? Electron Electron Gain/Lose Ion Element Configuration Configuration Electrons Formed (Atom) (Ion) S K I Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 5
Types of Bonding Ionic Solids: Assembly of cations and anions stacked together in a regular array. Not ote: Ionic solids are example of crystalline arrays in which the overall charge on an ionic solid is neutral. Ionic Compounds are represented with formula units (lowest ratio of types of atoms in the compound). Ionic Compound + - + CD - 2CD 3CD + - + - + - + - Covalent Compound AB 2 2AB 2 3AB 2 Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 6
Types of Bonding Steps to calculate the energy needed to form an ionic bond: Step 1: Standard states to gaseous single atom state Na(s) Na(g) 97 𝑙𝐾 𝑛𝑝𝑚 ½F 2 (g) F(g) 80. 𝑙𝐾 𝑛𝑝𝑚 Step 2: Both atoms have to form ions Na(g) Na + (g) + e - (g) 494 𝑙𝐾 𝑛𝑝𝑚 (ionization energy) F(g) + e - (g) F - (g) -323 𝑙𝐾 𝑛𝑝𝑚 ((-)electron affinity) Step 3: The ions need to come together to form a crystal (Lattice Energy) Na + (g) + F - (g) NaF(s) -923 𝑙𝐾 Not ote: When energy is 𝑛𝑝𝑚 released, the sign is negative Total Reaction because no work is needed to make the reaction happen. Na(s) + ½F 2 (g) NaF(s) 97 𝑙𝐾 𝑛𝑝𝑚 + 90. 𝑙𝐾 𝑛𝑝𝑚 + 494 𝑙𝐾 𝑛𝑝𝑚 + −323 𝑙𝐾 𝑛𝑝𝑚 + −923 𝑙𝐾 𝑛𝑝𝑚 = −575 𝑙𝐾 𝑛𝑝𝑚 Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 7
Types of Bonding What is holding ionic solids together? Coulombic Potential Energy −𝑎 𝑓2 = −𝑎2𝑓2 𝑎 1 𝑓 𝑎 2 𝑓 +𝑎 𝐹 𝑄,12 = 4𝜌𝜁∘𝑠12 = 4𝜌𝜁∘𝑒 4𝜌𝜁∘𝑒 Z 1 & Z 2 = charge of ions The total potential energy is the sum of all the potential energies 4𝜌𝜁∘ − 𝑎2𝑓2 𝑒 + 𝑎2𝑓2 2𝑒 − 𝑎2𝑓2 3𝑒 + 𝑎2𝑓2 4𝑒 ⋯ = − 𝑎2𝑓2 1 4𝜌𝜁∘𝑒 1− 1 2 + 1 3 − 1 𝐹 𝑄 = 4 +⋯ ote: 𝑚𝑜 2 = 1 − 1 2 + 1 3 − 1 Not 4 + ⋯ 𝑎2𝑓2 𝐹 𝑄 = −𝑚𝑜 2 4𝜌𝜁∘𝑒 Need to multiply by 2 to account for the other half of the line. 𝑎2𝑓2 𝐹 𝑄 = −2𝑚𝑜 2 4𝜌𝜁∘𝑒 Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 8
Types of Bonding Once neighboring ions come into contact they start to repel each other. ∗ ∝ 𝑓 − 𝑒 𝑒 ∗ Not ote: d* is a constant 𝐹 𝑄 that is commonly 𝑒 𝑒 ∗ = 1 d=0 𝑓 − max repulsion taken to be 34.5 pm 𝑒 𝑒 ∗ = 1 d>1 𝑓 − repulsion decreases The potential energy of an ionic solid is a combination of the favorable Coulombic interaction of the ions and the unfavorable exponential increase which results when the atoms touch. The ideal bond length occurs at the minimum potential energy. Energy Minimum Occurs: 𝐹 𝑞,𝑛𝑗𝑜 = − 𝑂𝐵 𝑎1𝑎2 1 − 𝑒 𝑒∗ 𝐵 4𝜌𝜁∘𝑒 Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 9
Types of Bonding Covalent Bond: A pair of electrons shared between two atoms (occurs between two non metals) Not ote: In covalent bond formation, atoms go as far as possible toward completing their octets by sharing electron pairs. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 10
Electronegativity Electronegativity (χ): The ability of an atom to attract electrons to itself when it is part of a compound Not ote: The atom with higher electronegativity has a stronger attractive power on electrons and pulls the electrons away from the atom with the lower electronegativity. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 11
Electronegativity Difference in Electronegativity Type of Bond > 1.8 Mostly Ionic 0.4-1.8 Polar Covalent < 0.4 Mostly Covalent 0 Non-polar Covalent The dividing line between ionic and covalent bonds is hazy. NaCl MgCl 2 AlCl 3 SiCl 4 PCl 3 S 2 Cl 2 Cl 2 ionic polar-covalent covalent Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 12
Electronegativity What makes covalent bonds partly ionic? Electric Dipole: A positive charge next to an equal but opposite negative charge. Electric Dipole Moment ( 𝝂 ): The magnitude of the electric dipole [units debye ( 𝐸 )]. ote: The dipole moment associated with H-Cl is about 1.1 𝐸 . Not Polar Covalent Bond: A covalent bond between atoms that have partial electric charges. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 13
Electronegativity What makes ionic bonds partly covalent? Polarizability ( 𝜷 ): The ease with which the electron cloud of a molecule can be distorted. As the cation’s positive charge pulls on the anion’s negative electrons, the spherical electron cloud of the anion becomes distorted in the direction of the cation. This causes the bond to have covalent bond properties. Not ote: The larger the anion the easier it is to distort the electron cloud. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 14
Lewis Structures Lewis Symbols: The chemical symbol of an element, with a dot for each valence electron. Step 1: Determine the number of valence e - from electron configuration. Step 2: Place 1 dot around the element for each valence electron. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 15
Lewis Structures Drawing Ionic Lewis Structures Step 1: Determine the electron configuration of the elements in the compound. Step 2: Determine the electron configuration of the ions that the elements form. Step 3: Draw the Lewis symbols for the ions. Do not forget to include charge. The cations should have no electrons around them and the anions should have 8 electrons around them. Step 4: Organize the Lewis symbols such that cations are next to anions. Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 16
Lewis Structures General Rules (Covalent Lewis Structures) All valence electrons of the atoms in the Lewis structures must be shown. Generally, electrons are paired. Except for odd electron molecules such as NO and NO 2 . Generally, each atom has 8 electrons in its valence shell with the exception of H which only needs 2 valance electrons. Multiple bonds (double and triple bonds) can be formed. Show atoms by their chemical symbols (ex. H) Show covalent bonds by lines (ex. F – F) Show lone pairs of electrons by pairs of dots (ex. :) Chapt pter er 13 13: Bondi nding: g: General eral Concep epts ts 17
Recommend
More recommend