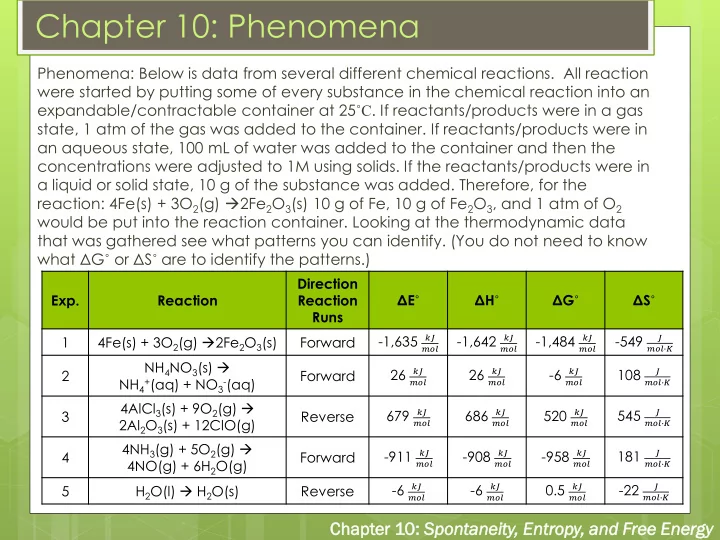

Chapter 10: Phenomena Phenomena: Below is data from several different chemical reactions. All reaction were started by putting some of every substance in the chemical reaction into an expandable/contractable container at 25 ˚C . If reactants/products were in a gas state, 1 atm of the gas was added to the container. If reactants/products were in an aqueous state, 100 mL of water was added to the container and then the concentrations were adjusted to 1M using solids. If the reactants/products were in a liquid or solid state, 10 g of the substance was added. Therefore, for the reaction: 4Fe(s) + 3O 2 (g) 2Fe 2 O 3 (s) 10 g of Fe, 10 g of Fe 2 O 3 , and 1 atm of O 2 would be put into the reaction container. Looking at the thermodynamic data that was gathered see what patterns you can identify. (You do not need to know what Δ G ˚ or Δ S ˚ are to identify the patterns.) Direction Exp. Reaction Reaction Δ E ˚ Δ H ˚ Δ G ˚ Δ S ˚ Runs -1,635 𝑙𝐾 -1,642 𝑙𝐾 -1,484 𝑙𝐾 -549 1 4Fe(s) + 3O 2 (g) 2Fe 2 O 3 (s) Forward 𝐾 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚∙𝐿 NH 4 NO 3 (s) 26 𝑙𝐾 26 𝑙𝐾 -6 𝑙𝐾 108 2 Forward 𝐾 NH 4 + (aq) + NO 3 - (aq) 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚∙𝐿 4AlCl 3 (s) + 9O 2 (g) 679 𝑙𝐾 686 𝑙𝐾 520 𝑙𝐾 545 3 Reverse 𝐾 2Al 2 O 3 (s) + 12ClO(g) 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚∙𝐿 4NH 3 (g) + 5O 2 (g) -911 𝑙𝐾 -908 𝑙𝐾 -958 𝑙𝐾 181 4 Forward 𝐾 4NO(g) + 6H 2 O(g) 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚∙𝐿 -6 𝑙𝐾 -6 𝑙𝐾 0.5 𝑙𝐾 -22 5 H 2 O(l) H 2 O(s) Reverse 𝐾 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚 𝑛𝑝𝑚∙𝐿 Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy
Chapter 10 Spontaneity, Entropy, & Free Energy o Entropy o ΔS of Physical Reactions o Isothermal Processes Big Idea: The change in free o 2 nd Law of Thermo energy of a reaction o Free Energy indicates whether a o Hess’s Law/ 3 rd Law of reaction is Thermo spontaneous. In any o Equilibrium spontaneous process there is always an increase in the entropy of the universe. 2
Entropy # of molecules # of ways of of left side arranging Entropy (S): Entropy is (microstates) 4 a measure of how 3 energy and matter 2 can be distributed in a 1 chemical system. 0 Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 3
Entropy In General: Entropy increases from solid to liquid to gas corresponding to an increase in positional probability. Entropy increases when you dissolve a solid in liquid corresponding to an increase in positional probability. The larger the volume the larger the positional probability and the greater the entropy (n constant). The larger the pressure the smaller the positional probability and the lower the entropy (n constant). The larger the molecule the larger the number of relative positions of the atoms resulting in a greater positional probability and a greater entropy. The higher the temperature the greater the range of energies, therefore the larger the entropy. Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 4
Entropy Student Question Predict which of the following reactions has a negative entropy change. I. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) II. NH 3 (g) + HCl(g) NH 4 Cl(s) III. 2KClO 4 (s) 2KClO 3 (s) + O 2 (g) a) II and III b) III c) II d) I e) I and II Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 5
Entropy Phase Change: The condition (for a given pressure, and temperature) at which two different phases are in dynamic equilibrium. Melting/Freezing Liquid/Solid Evaporation/Condensation Liquid/Gas Sublimation/Deposition Solid/Gas Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 6
Δ S of Physical Reactions Calculating ΔS for physical reaction X(s, T i ) X(g, T f ) Step 1: Calculate ΔS ∆𝑇 = 𝑛𝐷 𝑡𝑝𝑚𝑗𝑒 𝑚𝑜 𝑈 𝑁 𝑈 𝑗 to bring to melting point Step 2: Calculate ΔS n H fus S involved in fusion T Step 3: Calculate ΔS 𝑈 𝐶 ∆𝑇 = 𝑛𝐷 𝑚𝑗𝑟𝑣𝑗𝑒 𝑚𝑜 𝑈 𝑁 to bring to boiling point Step 4: Calculate ΔS n H vap involved in vaporization S T Step 5: Calculate ΔS 𝑈 𝑔 ∆𝑇 = 𝑜𝐷 𝑄 𝑏𝑡 𝑚𝑜 to bring to final temperature 𝑈 𝐶 Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 7
Δ S of Physical Reactions Student Question What is ΔS for 88.0 g of CO 2 undergoing the following reaction at constant pressure? CO 2 (s, 150. K) CO 2 (g, 195. K) Helpful Information: 𝑈 𝑡𝑣𝑐 = 195𝐿 , ∆𝐼 𝑡𝑣𝑐 = 25.2 𝑙𝐾 𝑛𝑝𝑚 , 𝐷 𝐷𝑃 2 (𝑡) = 1.07 𝐾 ∙𝐿 a) 24.9 𝐾 𝐿 b) 154 𝐾 𝐿 c) 233 𝐾 𝐿 d) 283 𝐾 𝐿 e) None of the above Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 8
Isothermal Processes Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 9
Isothermal Processes 2 Step Isothermal Expansion 6 Step Isothermal Expansion P P 1 1 Pressure Pressure P 1 2 P 1 2 P 1 4 P 1 4 V 2 V 4 V V 2 V 4 V 1 1 1 1 Volume 1 1 Volume ∞ Step Isothermal Expansion Reversible Process: A P process that can be 1 Pressure reversed by an infinitesimal change in a variable. P 1 2 P Not ote: In order for a reversible process to 1 4 occur the system must be at equilibrium during the entire process. V 2 V 4 V 1 1 1 Volume Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 10
Isothermal Processes Student Question Calculate the ΔS associated with a process in which 5.00 mol of gas expands reversibly at constant temperature T = 25°C from a pressure of 10.0 atm to 1.00 atm. a) 28,500 𝐾 𝐿 b) 95.7 𝐾 𝐿 c) -95.7 𝐾 𝐿 d) -28,500 𝐾 𝐿 e) None of the above Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 11
2 nd Law of Thermo 2 nd Law of Thermodynamics: A spontaneous change is accompanied by an increase in the total entropy of the system and its surroundings. ∆𝑇 𝑣𝑜𝑗 = ∆𝑇 𝑡𝑧𝑡 + ∆𝑇 𝑡𝑣𝑠 Not ote: The second law of thermodynamics applies to Δ S uni and not Δ S sys . So far we have only discussed Δ S sys . Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 12
Free Energy Can we relate spontaneity to a change in the system instead of the universe assuming we are at constant temperature and pressure? ∆𝐻 𝑡𝑧𝑡 = ∆𝐼 𝑡𝑧𝑡 − 𝑈∆𝑇 𝑡𝑧𝑡 Divide Through by T ∆𝐻 𝑡𝑧𝑡 ∆𝐼 𝑡𝑧𝑡 𝑈∆𝑇 𝑡𝑧𝑡 = − 𝑈 𝑈 𝑈 Δ S univ >0 then Δ G sys <0 ∆𝐻 𝑡𝑧𝑡 = −∆𝑇 𝑡𝑣𝑠 − ∆𝑇 𝑡𝑧𝑡 spontaneous process 𝑈 ∆𝐻 𝑡𝑧𝑡 Δ s univ <0 then Δ G sys >0 − = ∆𝑇 𝑣𝑜𝑗𝑤 non spontaneous process 𝑈 Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 13
Free Energy Student Question Hold the rubber band a short distance from your lips. Quickly stretch it and press it against your lips carefully (don’t hurt those delicate lips). Do you experience a warming or cooling sensation? Carefully release the rubber band and experience the sensation. Is stretching a spontaneous or a non-spontaneous process? What are the correct signs for ΔG, ΔH, and ΔS when you allow the rubber band to relax? Δ G Δ H Δ S a) – + + b) – – + c) + + – d) + – – Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 14
Hess’s Law / 3 rd Law of Thermo Hess’s Law: A reaction enthalpy/free energy/entropy is the sum of the enthalpies/free energies/entropies of any sequence of reactions (at the same temperature and pressure) into which the overall reaction can be divided. Things to remember: If you add reactions together, add ΔH/ΔG/ΔS. If you flip a reaction, flip the sign of ΔH/ΔG/ΔS. If you multiply a reaction by a constant, multiply ΔH/ΔG/ΔS by the same constant . Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 15
Hess’s Law / 3 rd Law of Thermo Student Question What is ΔG ° for SO 2 (g) + ½O 2 (g) SO 3 (g) given the following information? 2S(s) + 3O 2 (g) 2SO 3 (g) ΔG ° = -742 𝑙𝐾 𝑛𝑝𝑚 S(s) + O 2 (g) SO 2 (g) ΔG ° = -300. 𝑙𝐾 𝑛𝑝𝑚 a) -71 𝑙𝐾 𝑛𝑝𝑚 b) -442 𝑙𝐾 𝑛𝑝𝑚 c) -671 𝑙𝐾 𝑛𝑝𝑚 d) -1042 𝑙𝐾 𝑛𝑝𝑚 e) None of the above Chapt pter er 10: Sponta ntaneity eity, Entropy, and d Free e Energy 16
Recommend
More recommend