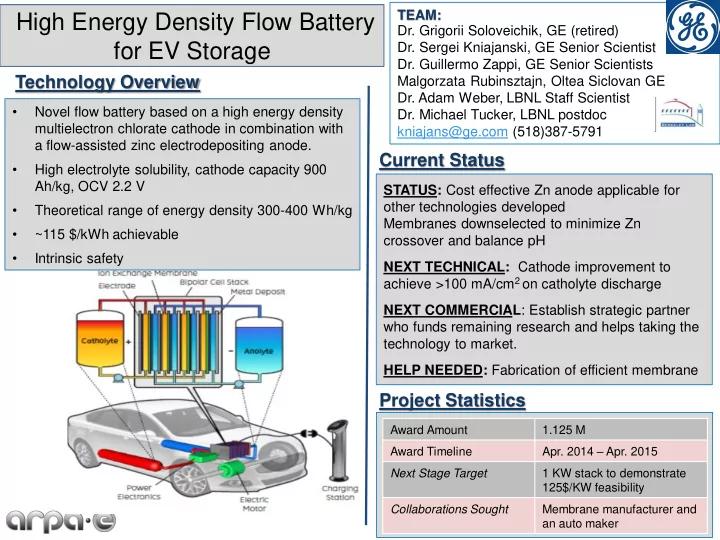

TEAM: High Energy Density Flow Battery Dr. Grigorii Soloveichik, GE (retired) for EV Storage Dr. Sergei Kniajanski, GE Senior Scientist Dr. Guillermo Zappi, GE Senior Scientists Technology Overview Malgorzata Rubinsztajn, Oltea Siclovan GE Dr. Adam Weber, LBNL Staff Scientist • Novel flow battery based on a high energy density Dr. Michael Tucker, LBNL postdoc multielectron chlorate cathode in combination with kniajans@ge.com (518)387-5791 a flow-assisted zinc electrodepositing anode. Current Status • High electrolyte solubility, cathode capacity 900 Ah/kg, OCV 2.2 V STATUS: Cost effective Zn anode applicable for other technologies developed • Theoretical range of energy density 300-400 Wh/kg Membranes downselected to minimize Zn • ~115 $/kWh achievable crossover and balance pH • Intrinsic safety NEXT TECHNICAL: Cathode improvement to achieve >100 mA/cm 2 on catholyte discharge NEXT COMMERCIAL : Establish strategic partner who funds remaining research and helps taking the technology to market. HELP NEEDED: Fabrication of efficient membrane Project Statistics Award Amount 1.125 M Apr. 2014 – Apr. 2015 Award Timeline 1 KW stack to demonstrate Next Stage Target 125$/KW feasibility Membrane manufacturer and Collaborations Sought an auto maker
Technology description Initial challenges Anode Zn concentration 3 M Corrosion stability in acidic media Zn dense plating vs slurry Cathode Slow discharge kinetics, electrocatalyst Faradaic efficiency Overpotential pH control - + 6 H + + 6e - Cl - + 3 H 2 O ClO 3 Zn 2+ + 2e - Zn 0 Membrane High H + conductivity at low M + crossover GE proprietary, patent pending System integration High energy efficiency Cycleability Water balance pH control
Anolyte development • Several buffered, cost effective anode formulations developed with Zn concentration 2.5-3.5 M at 0% SoC • pH well maintained over cycling • > 97% Zn deposition efficiency on 150 mA/cm 2 charge • < 3% Zn non-coulombic loss on 150 mA/cm 2 discharge Morphology of Zn deposition • Flat Zn deposition morphology on 150 mA/cm 2 charge • Demonstrated on 40 cm 2 Zn electrode in a flow cell • Efficiency mostly affected by buffer Electrolyte 1 composition rather than by pH 50 % SoC, 150 mA/cm 2 Electrolyte 2 0 % SoC, 500 mA/cm 2 • Could be used in other technologies
Cathode development Current density on different electrodes • - to Cl - reduction is Slow kinetics of ClO 3 the biggest challenge • pH increase is not favorable • Chronopotentiometry used for screening potential catalytically active electrodes and reaction conditions • Promising candidates have been identified pH effect on current density @ 0.5V 25 8 Current density, mA/cm 2 7 • Results have recently improved to ~100 20 6 mA/cm 2 at small scale 5 15 pH Current density 4 Catholyte pH 10 • Electrodes with enhanced surface area 3 Anolyte pH 2 electrode may help in reaching the 5 1 target 0 0 6000 7000 8000 9000 10000 Reaction time, s
Membrane Selection ASR Zn Crossover Cost Conductivity (Ohm- Rate $/m2 (mS/cm) cm2) (mg/h/cm2/M) Daramic Lead-acid separator 4-6 16 1.6 5.4 Millipore PES Filtration 70-200 59 0.2 10.4 NR211 As-received 350-1000 3 0.8 0.03 NR211 Boiled 350-1000 16 0.2 5.5 Tradeoff between conductivity and crossover Range of PEM and Microporous overlap Not enough difference for downselection between PEM and Microporous Microporous significantly less expensive • Measured conductivity and crossover for range of PEM and microporous separators • Downselected to NR211, Millipore PES, and Daramic for further testing
System integration 2.0 1.8 Chlorate ca cathode with H 2 anode 1.6 H 2 /GDL/Pt-C/NR211/Ti 1.4 mesh/GDLx3/3M NaClO 3 with HCl, no 1.2 catalyst Voltage (V) Performance stable drawing current 1.0 3M NaClO3 40 mA/cm 2 at 1.0 V for at least 20 min 0.8 HCl 0.6 0.4 0.2 0.0 0 20 40 60 80 100 120 Flow cell – 82 cm 2 membrane area 2 ) Current Density (mA/cm Current(A), Voltage(V) vs. Test_Time(s) • Chlorine evolution at low pH 1.5 2.5 1-001 Current(A) 1-001 Voltage(V) 1 • Shallow cycles demonstrated at 2 higher pH in developed electrolyte 0.5 1.5 Current(A) Voltage(V) • Product analysis underway 0 0 5000 10000 15000 20000 25000 1 • Deep cycling and pH control to be -0.5 demonstrated 0.5 -1 Chlorate anode with zinc c anode -1.5 0 Test_Time(s)
Recommend
More recommend