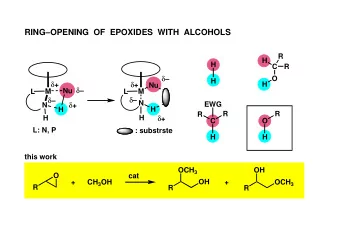
DEHYDROGENATIVE TRANSFORMATION OF ALCOHOLS Ar Ar Ar Ar P P - PowerPoint PPT Presentation
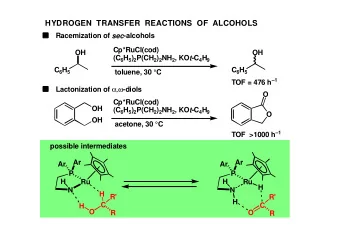
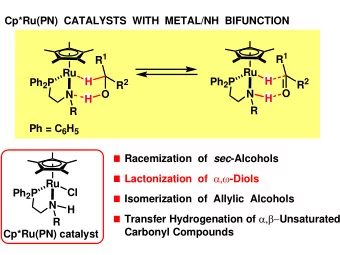
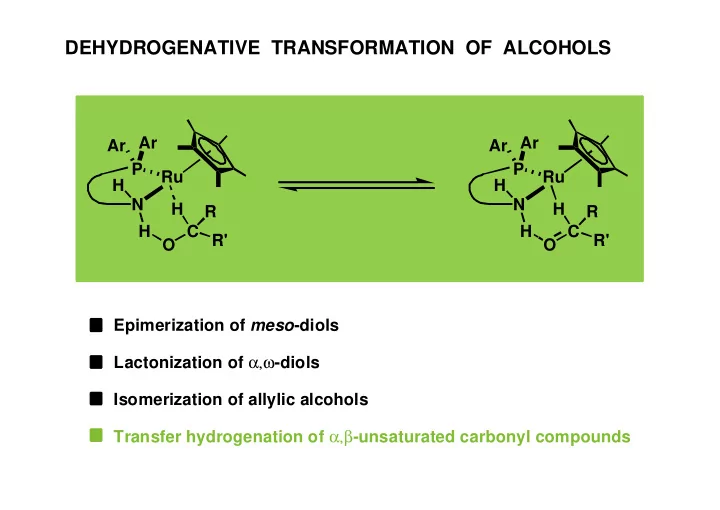
DEHYDROGENATIVE TRANSFORMATION OF ALCOHOLS Ar Ar Ar Ar P P Ru Ru H H N N H H R R H C R' H C R' O O Epimerization of meso -diols Lactonization of , -diols Isomerization of allylic alcohols Transfer hydrogenation of
DEHYDROGENATIVE TRANSFORMATION OF ALCOHOLS Ar Ar Ar Ar P P Ru Ru H H N N H H R R H C R' H C R' O O Epimerization of meso -diols Lactonization of α,ω -diols Isomerization of allylic alcohols Transfer hydrogenation of α,β -unsaturated carbonyl compounds
CATALYTIC ISOMERIZATION OF ALLYLIC ALCOHOLS Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] R 2 R 2 OH O KO t -Bu R 4 R 3 R 4 R 3 toluene 30 °C, 1 h R 1 R 1 alcohol:Ru:KO t- Bu = 100:1:1 > 99% yield R 2 OH R 4 R 3 R 1 R 2 O Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] + Ru Ru + R 4 R 3 H Ph 2 P KO t -Bu NH Ph 2 P NH 2 R 1 R 2 O R 4 R 3 R 1 日本化学会第8 1 春季年会3 G 4 1 3 ( 2 0 0 2 )
ENONE EXCHANGE REACTION Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] OH O KO t -Bu + C 6 D 6 Ph 0.5 M, 30 °C, 1 h >99% conv. alcohol:enone:Ru:KO t- Bu = 100:100:1:1 O O Ph + O O Ph Intermolecular Intramolecular 24 % 76 %
TRANSFER HYDROGENATION OF ACTIVATED OLEFIN WITH ALCOHOL RuCl 2 (PPh 3 ) 3 , O O OH O 0.25 mol% + 92% + PhCH(OH)CH 3 , Ph Ph Ph Ph Ph Ph 180 °C, 1 h Sasson et al. J. Org. Chem. , 1975, 40 , 1887 O O [RuH(( S )-binap) 2 ]PF 6 , OH O 2 mol% + + >99% HO R HO R R 2 R 2 EtOH or 2-propanol, reflux, 24 h R 1 R 1 R 1 = CH 2 COOH R 1 = NHCOCH 3 R 2 = H or CH 3 Saburi et al.Tetrahedron Lett. , 1992, 33 , 5783 [Ir(cod)Cl] 2 /dppp/ O OH O O Cs 2 CO 3 , 2 mol% + + R 1 R 2 R 3 R 1 R 2 R 3 Ph Ph toluene R 1 = CH 3 , R 2 = CH 3 , R 3 = CH 3 80 °C, 4 h 93% R 1 = OBn, R 2 = Ph, R 3 = H 150 °C, 72 h 92% Sakaguchi et al. J. Org. Chem. , 2001 , 66 , 4710 Edwards et al. Angew. Chem. Int. Ed. , 2002, 41, 4740
TRANSFER HYDROGENATION OF ENONEs USING 2-PROPANOL Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] R 2 R 2 OH O O O KO t -Bu + + toluene R 3 R 3 R R 30 °C, 1 h R 1 R 1 enone:alcohol:Ru:KO t -Bu = 100:100:1:1 [enone] = 0.5 M O O Ph >99% yield >99% O O O 47% 52% 38% endo : exo = >99:<1
TRANSFER HYDROGENATION OF α,β− UNSATURATED ESTERs WITH ALCOHOL Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] R 2 R 2 OH O KO t -Bu ROCO + ROCO + R 3 toluene R 3 0.5 M, 30 °C, 1 h R 1 R 1 ester:Ru:KO t -Bu = 100:1:1 CH 3 OCO EtOCO BnOCO EtOCO CH 3 OCO >99% yield >99% >99% 41% EtOCO EtOCO n -BuOCO EtOCO CO 2 Et 0% >99% 54% 4% 4% O O O O 2-propanol large excess 10 equiv. 54 % 13 %
ISOTOPE LABELING EXPERIMENT β α BnOCO BnOCO D Cp*RuCl[Ph 2 P(CH 2 ) 2 NH 2 ] D KO t -Bu + + toluene O OD 0.5 M, 30 °C, 2 h, CD 3 CD 3 CD 3 CD 3 D ester:alcohol:Ru:KO t -Bu = 100:100:1:1 87% conv. α position; 0.86 D loss of deuterium ! β position; 1.00 D determined by 1 H & 2 H NMR. C 6 D 6 as an internal standard ( 2 H NMR)
DISTRIBUTION OF DEUTERIUM 1.00 D cis ; 0.31 D trans ; 0.31 D 0.86 D BnOCO BnOCO BnOCO + H H BnOCO β -hydride D H BnOCO elimination H [Ru] D [Ru] [Ru] H D BnOCO H
TRANSFER HYDROGENATION OF α,β -UNSATURATED CARBONYL COMPOUNDs OH O Ru Ru H Ph 2 P NH Ph 2 P NH 2 EWG EWG H Chemoselective reduction of activated olefin [Ru] Insertion mechanism via ruthenium-C intermediate EWG
Recommend
More recommend
Explore More Topics
Stay informed with curated content and fresh updates.