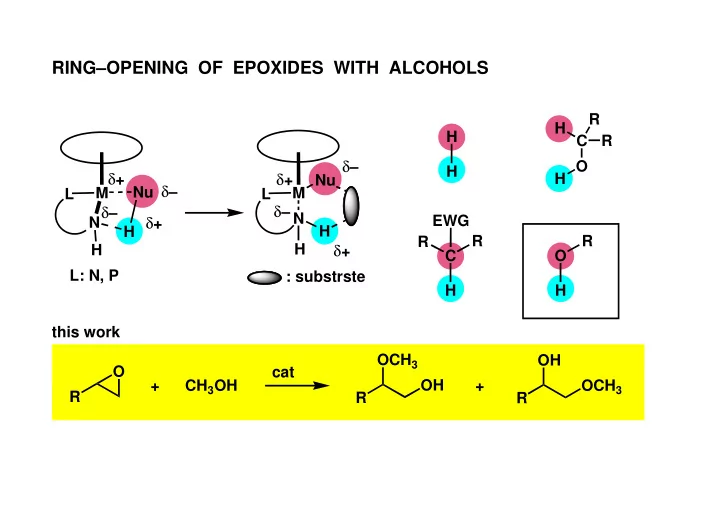

RING–OPENING OF EPOXIDES WITH ALCOHOLS R H H C R δ – O H δ + δ + H Nu δ – Nu M M L L δ – δ – δ + N EWG N H H R R R δ + H H C O L: N, P : substrste H H this work OCH 3 OH O cat + CH 3 OH OH + OCH 3 R R R
REACTION OF STYRENE OXIDE WITH METHANOL OH OCH 3 O OCH 3 OH cat + CH 3 OH + CH 3 OH 50 °C, 24 h A B epoxide:CH 3 OH:cat = 50:500:1 Ru cat: Ts product N N yield, % conv, % cat A:B Ru Ru N Cl N arene–Ru 50 29 90:10 H H 2 Cp*RuCl/KOH 31 31 82:18 arene–Ru complex Cp*RuCl complex KOH 82 33:67 97 Ts = p -CH 3 C 6 H 4 SO 2
REACTION OF STYRENE OXIDE WITH ALCOHOLS OR OH Ru cat O OH + OR + ROH C 6 H 5 C 6 H 5 C 6 H 5 ROH 50 °C, 24 h A B epoxide:ROH:cat = 50:500:1 Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) yield, % byproducts: ROH A B byproducts OH CH 3 OH 26 1 34 C 6 H 5 CH 3 CH 2 OH 22 1 25 OH (CH 3 ) 3 COH <1 <1 0 C 6 H 5
LIMITATION O O C 6 H 5 C 6 H 5 reactive inert O O O C 6 H 5 inert inert
REACTION OF rac -STYRENE OXIDE WITH METHANOL OH OCH 3 O OH OCH 3 * * ( S,S )-Ru cat + + CH 3 OH CH 3 OH 50 °C, 6 h A B 95:5 racemate 34% conv 20% yield epoxide:CH 3 OH:cat = 50:500:1 68% ee ( R ) ( S,S )-Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) Ts C 6 H 5 N Ru N C 6 H 5 H
REACTION OF ( S )-STYRENE OXIDE WITH METHANOL OCH 3 OH O OH OCH 3 S Ru cat R * + + CH 3 OH CH 3 OH 50 °C, 24 h >99% ee A B epoxide:CH 3 OH:cat = 50:500:1 recovered epoxide product (A) yield, % ee, % (config) yield, % ee, % (config) Ru cat Ru[( S,S )-Tsdpen]( p -cymene) 6 ( R ) 40 ( R ) 9 68 Ru[( R,R )-Tsdpen]( p -cymene) 97 ( S ) 76 ( R ) 58 31 Ru(Ts-en)( p -cymene) 95 ( S ) 63 ( R ) 67 27
RACEMIZATION OF ( S )-STYRENE OXIDE O O chiral Ru cat S solvent 50 °C, 24 h epoxide:solvent:chiral cat = 50:500:1 recoverd epoxide yield, % ee, % (config) chiral Ru cat solvent chiral Ru cat: Ts CH 3 OH S,S 9 5 ( R ) C 6 H 5 N * R,R CH 3 OH 58 97 ( S ) Ru S,S (CH 3 ) 3 COH 95 14 ( R ) N C 6 H 5 * H (CH 3 ) 3 COH R,R 97 99 ( S ) S,S C 6 H 6 >99 57 ( R ) Ts = p -CH 3 C 6 H 4 SO 2 >99 20 ( R ) R,R C 6 H 6
cis – trans ISOMERIZATION OF STILBENE OXIDE O O ( S , S )-Ru cat CH 3 OH 50 °C, 24 h 14% conv cis trans 14% yield 19% ee O O ( S , S )-Ru cat CH 3 OH 50 °C, 24 h cis (±)- trans no reaction epoxide:CH 3 OH:Ru= 50:500:1 ( S , S )-Ru cat: Ru[( S,S )-Tsdpen]( p -cymene)
SUMMARY OCH 3 OCH 3 O[Ru] OH OH CH 3 OH CH 3 OH S R S N 2 S N 1 CH 3 OH O[Ru] O[Ru] R
Recommend
More recommend