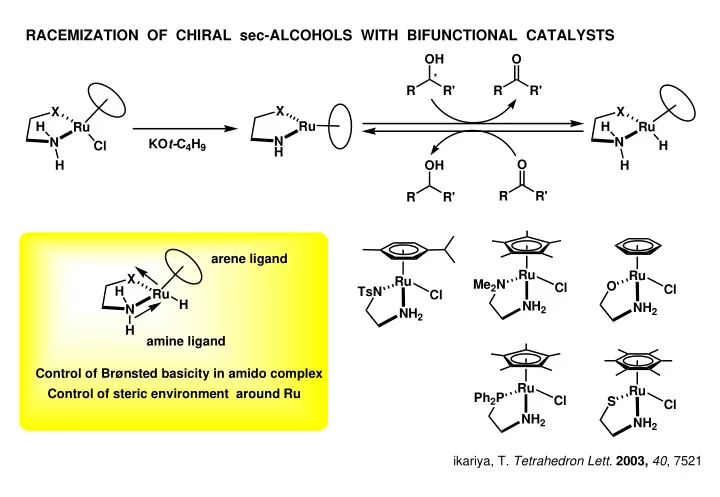

RACEMIZATION OF CHIRAL sec-ALCOHOLS WITH BIFUNCTIONAL CATALYSTS OH O * R R' R R' X X X Ru H Ru H Ru N N N KO t- C 4 H 9 Cl H H O H OH H R R' R R' arene ligand Ru Ru X Ru Me 2 N O Cl Cl H TsN Ru Cl H NH 2 NH 2 N NH 2 H amine ligand Control of Brønsted basicity in amido complex Ru Ru Control of steric environment around Ru Ph 2 P Cl S Cl NH 2 NH 2 ikariya, T. Tetrahedron Lett. 2003, 40 , 7521
RACEMIZATION OF sec-ALCOHOLS: A KEYSTEP IN DYNAMIC KINETIC RESOLUTION OF CHIRAL RACEMIC sec – ALCOHOLS O OH slow O R acyl donor + R 1 R 2 lipase R 1 R 2 Ru cat O OH fast O R + acyl donor lipase R 1 R 2 R 1 R 2 Ph Ph O O Ph Ph Ph Ph Ph H NH Ph Ph Ph Ph Ph Ph Ph Ph Ph H X = Cl Ph Ru Ru Ru Ru Br OC Cl OC X OC CO CO I OC CO CO Shvo (1986) + KO t- Bu + KO t- Bu B äckvall (1999) Park (2002) B äckvall (2004) Casey (2001)
A POSSIBLE MECHANISM OF RACEMIZATION BY Ru –AMINE COMPLEXES OH R R' R R' KO t- C 4 H 9 Ru Ru R' R Ru Ru X X X Cl H H X NH NH 2 HN or NH O O H H diastereomeric OH OH + R R' R R' O R R' R R' Ru R' Ru R Ru X X X H H H + HN O HN O HN H H H O R R'
EFFECTIVE CHELATE AMINE LIGAND FOR Ru OH OH [RuCl 2 (hmb)] 2 amine ligand KO t- C 4 H 9 M toluene, 30 °C NH X 24 h >99% ee S/C = 100 postulated catalyst other amine ligand amine ligand ON chelate HO NH 2 HO NH 2 HO NH 2 H O NH 2 O O O S 14 >99 88 86 ee, % HO NH 2 HO NH 2 86 58 amine ligand SN chelate HS NH 2 HS NH 2 HS NH 2 PhS NH 2 HS NMe 2 TsN NH 2 TsN NH 2 H H 7 >99 >99 91 ee, % 99 76 >99
LIGAND EFFECT: ARENE LIGANDS R n [RuCl 2 (arene)] 2 OH OH HS(CH 2 ) 2 NH 2 Cl Cl KO t- C 4 H 9 Ru Ru R n Cl toluene, 30 °C Cl 24 h >99% ee S/C = 100 [RuCl 2 (arene)] 2 arene ligand 99 94 99 ee, % arene ligand ee, % 92 68 7
EFFECTIVE CHELATE AMINE LIGAND FOR Rh or Ir OH OH [Cp*MCl 2 ] 2 amine ligand M KO t- C 4 H 9 X NH toluene, 30 °C 24 h >99% ee postulated catalyst S/C = 100 amine none HO NH 2 TsN NH 2 HS NH 2 Metal H Rh >99% ee >99 >99 97 Ir 99 97 98 99
SYNTHESIS OF PREFORMED CATALYST PRECURSORS Cl S [RuCl 2 (hmb)] 2 N CH 3 ONa Ru KOH (excess) NH 2 H Ru Ru S S 2-propanol THF H HS NH 2 NH 2 (Ru:SN=2:5) 78% yield 68% yield KO t- C 4 H 9 P-1 (#2) 2-propanol P-1 (#2) R1 = 0.120 R1 = 0.079 wR2 = 0.318 wR2 = 0.188 NH 3 Cl 2 H 2 N Cl S 1/2 Ru Ru S P-1 (#2) R1 = 0.057 91% yield wR2 = 0.148
PREPARATION OF GROUP IX SN COMPLEXES NH 3 Cl 2 H 2 N Cl KO t- C 4 H 9 S Rh Rh 2 [Cp*RhCl 2 ] 2 + HS NH 2 2-propanol S 1 H NMR ( δ , CDCl 3 ):1.61 (s, 15H, Cp*) 1.80 (s, 15H, Cp*) ESI-MS: m/z 663 [M-H] + NH 3 Cl 2 H 2 N Cl KO t- C 4 H 9 S [Cp*IrCl 2 ] 2 2 Ir Ir + 2-propanol HS NH 2 S 1 H NMR ( δ , CDCl 3 ): 1.60 (s, 15H, Cp*) 1.80 (s, 15H, Cp*) ESI-MS: m/z 841 [M-H] +
CATALYTIC ACTIVITY OF ISOLABLE RuSN COMPLEXES OH OH Ru cat KO t- C 4 H 9 toluene, 30 °C 6 h >99% ee S/C = 100 NH 3 Ru cat: Cl 2 H 2 N Cl S Ru Ru S 6% ee Cl S N H Ru NH 2 Ru Ru S S H NH 2 99% ee 99% ee
RACEMIZATION WITH PREFORMED Ru, Rh, Ir COMPLEXES OH OH metal cat KO t- C 4 H 9 (3 eq) toluene, 30 °C 24 h >99% ee S/C = 100 metal cat : NH 3 NH 3 NH 3 Cl 2 Cl 2 Cl 2 H 2 N H 2 N H 2 N Cl Cl Cl S S S Rh Ir Ru Rh Ir Ru S S S 6% ee 99 64 [RuCl 2 (hmb)] 2 + [Cp*RhCl 2 ] 2 + [Cp*IrCl 2 ] 2 + HS NH 2 HS NH 2 HS NH 2 36 98 >99
A POSSIBLE ROUTE FOR GENERATION OF ACTIVE CATALYSTS NH 3 Cl 2 Cl 2 H 2 N Cl S NH 2 S NH Ru S Ru Ru Ru Ru Ru base base S H 2 N S S HN 18e O OH R 1 R 2 R 1 R 2 Ru Ru S H NH S NH 2 O OH 16e R 1 R 2 R 1 R 2
SYMMETRIC DIMER Cl 2 S NH 2 [RuCl 2 (hmb)] 2 2 + Ru Ru CH 2 Cl 2 S 50 °C H 2 N HS NH 2 18% yield P2 1 / n (#14) R1 = 0.060 wR2 = 0.176 Ru(1) –S(1) 2.411 Bond lengths (Å) Ru(1) –S(2) 2.418 Ru(1) –N(1) 2.183 Bond angles (°) S(1) –Ru(1) –S(2) 82.38 S(1) –Ru(1) –N(1) 78.00 S(2) –Ru(1) –N(1) 80.30 1 H NMR (CD 3 OD) : δ 1.78 (s, 36H) δ 7.19 (t, 2H) δ 7.32 (t, 2H) δ 6.48 (m, 4H)
TRANSFER HYDROGENATION O OH metal cat OH O KO t- C 4 H 9 (3 eq) + + 30 °C 1 h [ketone] = 1.0 M in 2-propanol metal cat: NH 3 NH 3 NH 3 Cl 2 Cl 2 Cl 2 H 2 N H 2 N H 2 N Cl Cl Cl S S S Ru Rh Ir Ru Rh Ir S S S 83% 33 84 [RuCl 2 (hmb)] 2 + [Cp*RhCl 2 ] 2 + [Cp*IrCl 2 ] 2 + HS NH 2 HS NH 2 HS NH 2 36 51 71
SUMMARY Synthesis of new unsymmetric S-bridged complexes & symmetric dimer NH 3 NH 3 Cl 2 Cl 2 Cl 2 H 2 N H 2 N Cl Cl S NH 2 Ru S S Ru Ru M Ru M S H 2 N S S M = Rh, Ir OH O NH 3 Cl 2 H 2 N R R R R Cl S base M M M M S S H S NH NH 2 OH O R R R R Reactivity order: (hmb)Ru > Cp*Ir > Cp*Rh
Recommend
More recommend