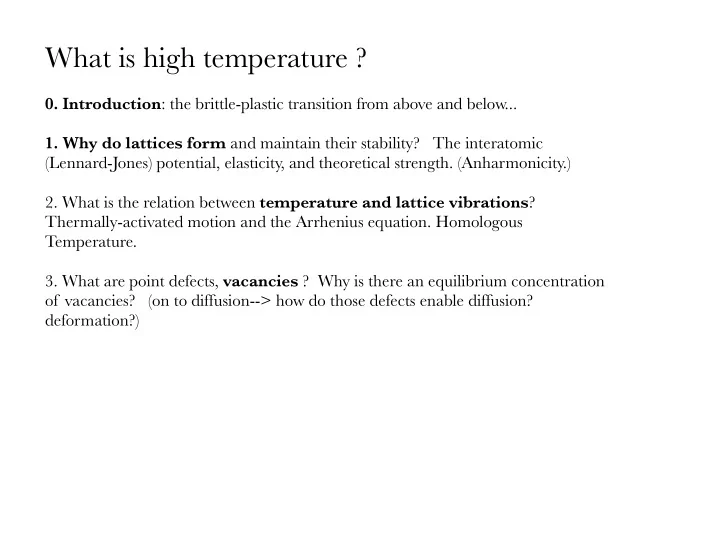

What is high temperature ? 0. Introduction : the brittle-plastic transition from above and below... 1. Why do lattices form and maintain their stability? The interatomic (Lennard-Jones) potential, elasticity, and theoretical strength. (Anharmonicity.) 2. What is the relation between temperature and lattice vibrations ? Thermally-activated motion and the Arrhenius equation. Homologous Temperature. 3. What are point defects, vacancies ? Why is there an equilibrium concentration of vacancies? (on to diffusion--> how do those defects enable diffusion? deformation?)
As the temperature starts to rise in the crust, towards the brittle- plastic transition, what happens to the material? What deformation processes become increasingly possible? Or the inverse, coming from below, as the temperature starts to cool, what happens to the deformation mechanisms?
Creep processes are irreversible, constituting “yield”, or a deviation from the linear elastic part of the stress strain curve. The smallest scale irreversible process is diffusion, which becomes possible when thermal motion/energy is vigorous enough. The higher the temperature, the faster it is...
at “high temperature” these stress concentrations cannot develop, because they are relaxed by diffusion or other “creep” processes... at high pressure, the cracks cannot open, 1) a material with “flaws” rocks deform by slower smoother or Griffith cracks processes than in the schizosphere...
1. Why do lattices form and maintain their stability? The interatomic (Lennard-Jones) potential, elasticity, and theoretical strength. (Anharmonicity.) ~1920 Bragg & son (W.L. and W. H. ) -> x-ray scattering in metals produced diffraction patterns : a crystal lattice ! ~ 1926, the theoretical strength crisis ! (Griffith-- brittle glass) (Frenkel-- ductile metals)
Interatomic Potential repulsive potential two atoms b = 2r r x attractive potential = coulombic/ionic/metallic/van der wals attractive repulsive potential = potential harmonic approximation pauli exclusion (overlapping orbitals) repulsion �� x � 6 � � x � 12 U ia = E aa − r r attraction the depth of the well is proportional gas solid http://en.wikipedia.org/wiki/John_Lennard-Jones “Anharmonicity is the deviation of a system from being a harmonic oscillator.” - wikipedia
Periodic model, theoretical strength (Frenckel derivation... ) b b %!! a $!! -1/340/56-2785/31/-09:7; - E = Fx #!! F = dE ! dx ! #!! τ = dF ! $!! dA ! %!! ! !"# !"$ !"% !"& !"' !"( !") !"* !"+ # ,-./0123 τ = τ max sin 2 π x * & <7#! b τ ≈ τ max 2 π x b $ τ = µ γ ./43.. ! γ ≈ x a ! $ τ max = µ b 2 π a ! & ! !"# !"$ !"% !"& !"' !"( !") !"* !"+ # τ max ≈ µ ,-./0123 2 π = shear modulus
high temperature high temperature high temperature 2. What is the relation between temperature and lattice vibrations ? Thermally-activated motion and the Arrhenius equation. ( )=( )( ) k = A exp − E a # collisions total # probability of => reaction collisions rxn success RT � 1 � − E a energy ln( k ) = T + ln( A ) barrier T thermal � � ∂ ln k energy E a = − R ∂ 1 T P low temperature - E a /R ln (k) 1 /T high temperature http://en.wikipedia.org/wiki/Arrhenius_equation
Oxides low temperature Salts high temperature Karato, p 179
so what do we define as “high temperature”? ! "'"& 345146,*-378)91,)-:.7641,-.)15;<=>> ! "'"! ! !"!% 789(8:1/27;<.=(1.2>3;:8(123.(9?,@A(A ! !"!+ ! "'"# increasing activation ! !"!* energy ! "'"$ ! !"!) ! !"$ ! "'% ! !"$% k = A exp − E a !"# $ $"# % %"# & &"# $,-./0.12341.5( ° (6 ! $ ( ! "'%& ! & RT '($! � � !"" #"" $"" %""" %&"" %!"" %#"" %$"" &""" ()*+),-./,)01 ° 121 => a fraction of the melting temperature (of the pure phase), called the homologous temperature, T/T m
3. What are point defects, vacancies ? Why is there an equilibrium concentration of vacancies? (on to diffusion--> how do those defects enable diffusion? deformation?) silicon metal, atomic force microscopy ~1 nm http://www.omicron.de/
2-D 3-D... random walks...
The equilibrium concentration of vacancies Maxwell-Boltzmann $!! Statistical Thermodynamics: S conf = − k B ln W � ( N + n v )! � #!! 1.13:/*+;<=708 increasing T W = N ! n v ! ! G ( T, P, N, n v ) = G 0 ( T, P ) + n v g f v − k B T ln W ! #!! � ( N + n v )! � G ( T, P, N, n v ) = G 0 ( T, P ) + n v g f v − k B T ln N ! n v ! ! $!! � ∂ G ! %!! � v − k B T ∂ = g f ln W ! !"!!# !"!!$ !"!!% !"!!& !"!' ∂ n v ∂ n v ( T,P ) ( ) *+),-,.-/+-0.-1.23,240.+5613+70819+ � ∂ G � � ( N + n v )! � v − k B T ∂ = g f ln ∂ n v ∂ n v N ! n v ! ( T,P ) ( ) *+EF"+),-,.-/+-0.-"+5G=70819 � ∂ G ! A � � � n v = g f v − k B T ln ∂ n v N + n v ( T,P ) � ∂ G � = g f ! % v − k B T ln ( X v ) ∂ n v ( T,P ) � � ! > � � ∂ G where = 0 ∂ n v � � ! & � � g f ln v X eq v = exp > & ? '! '' '# k B T '=B17613,2C31*+D ! $ @+'!
What is the melting temperature ? 12 atoms at some thermal state, there is enough kinetic (thermal) energy and a large enough vacancy concentration that the lattice begins to break down... 12 atoms 12 atoms X v = (1/12) 3 ~ 5.7e-4 at 1340 K in gold metal. so what is “high temperature” from a material perspective? when creep (i.e. diffusion) can be fast enough to relax any stress concentrations... T/T m >0.5-0.6... (but of course, this depends on the time scale of the stress change...)
SUMMARY !!! 1. Elasticity is small strain, not sensitive to activation energy, but very sensitive to lattice spacing. 2. The theoretical strength is much larger than observed strength... 3. Temperature increases the probability that atoms can jump over the activation energy “barrier”... Arrhenius relation 4. Vacancies exist and have equilibrium concentrations (i.e. they are not “imperfection”) that are exponential functions of temperature. 5. “Melting” can be thought of as a critical concentration of vacancies... 6. “High T” is some fraction of the melting temp, rule of thumb: T/T m > 0.5
creep processes are irreversible, constituting “yield”, or a deviation from the linear elastic part of the stress-strain curve. the smallest scale irreversible process is diffusion, which becomes possible when thermal motion/energy is vigorous enough. The higher the temperature, the faster it is...
at “high temperature” these stress concentrations cannot develop, because they are relaxed by diffusion or other “creep” processes... at high pressure, the cracks cannot open, 1) a material with “flaws” rocks deform by slower smoother or Griffith cracks processes than in the schizosphere...
Temperature, °C The Structure and Physical Properties of the Earth’s Crust, Geophysical Monograph 14, editor John G. Heacock, pp. 169 – 184, 1971. The Thermal Structure of the Continental Crust DAVID D . BLACKWELL measured heat flow at surface estimated temperatures at a depth of 6 kilometers http://smu.edu/geothermal/heatflow/ http://www1.eere.energy.gov/geothermal/geomap.html
Theoretical strength • Anharmonic model of the atomic bond
Stress concentrations
Brittle-plastic transition
Experimental results of brittle-plastic transition
Recommend
More recommend