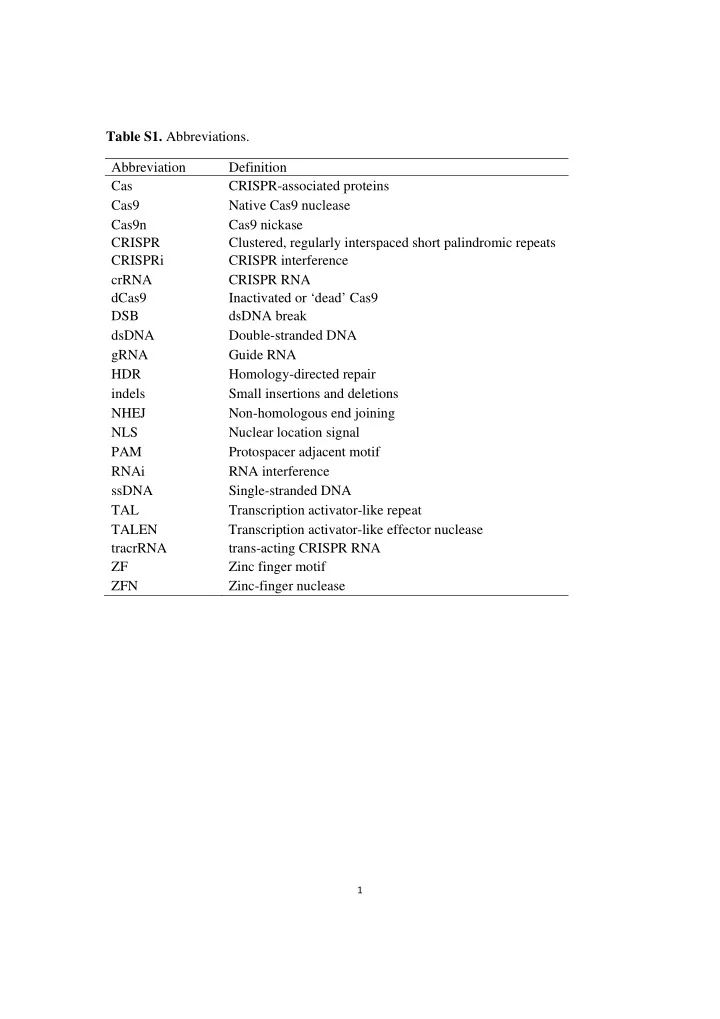

Table S1. Abbreviations. Abbreviation Definition Cas CRISPR-associated proteins Cas9 Native Cas9 nuclease Cas9n Cas9 nickase CRISPR Clustered, regularly interspaced short palindromic repeats CRISPRi CRISPR interference crRNA CRISPR RNA dCas9 Inactivated or ‘dead’ Cas9 DSB dsDNA break dsDNA Double-stranded DNA gRNA Guide RNA HDR Homology-directed repair indels Small insertions and deletions NHEJ Non-homologous end joining NLS Nuclear location signal PAM Protospacer adjacent motif RNAi RNA interference ssDNA Single-stranded DNA TAL Transcription activator-like repeat TALEN Transcription activator-like effector nuclease tracrRNA trans-acting CRISPR RNA ZF Zinc finger motif ZFN Zinc-finger nuclease 1
Table S2. Application of Cas9-based tool in genome editing and transcriptional control. Application Strategies Mutation types Organisms/cell types Target loci Efficiency Referen ces Genome Cas9 and NHEJ-induced indels Mouse Th 4.8-27% 1 editing tracrRNA:crRNA Human HEK 293FT cells EMX1, PVALB 1.5-21% 1 duplex Rice callus cells CAO1 2 DNA insertion Streptococcus pneumoniae 100% 3 srtA, bgaA, ermAM Escherichia coli rpsL 96% 3 DNA deletion Human HEK 293FT cells EMX1, PVALB 1.6% 1 Cas9 and gRNA NHEJ-induced indels Human K562, HEK 293FT and iPS AAVS1, DNMT3a/b, CLTA, CCR5, C4BPB 2-33% 4-7 cells Zebrafish embryos fh, apoea, th1, rgs4, tph1a, gsk3b, gata4/5, 24.1-59.4% 7, 8 drd3, tia1l, etsrp Drosophila preblastoderm embryos Yellow, rosy 7.7-75% 9 Caenorhabditis elegans germ line unc-119, dpy-11, dpy-13, klp-12, 0.5-80.3% 10, 11 Y61A9LA.1, EGFP, lin-5, rol-1 Xenopus tropicalis embryos 100% 12 tyr, six3 Saccharomyces cerevisiae CAN1 0.01-0.07% 13 Arabidopsis protoplast and seedlings AtPDS3,AtFLS2, AtRACK1b, AtRACK1c, 1.1-5.6% in protoplast; 2.7% 14, 15 GFP in leaf agroinfiltration Rice protoplast and callus cells OsMPK2/5, GFP, OsPDS, LAZY1, CAO1, 3-38% in protoplast 2, 15- Os02g23823, OsBADH2 17 Wheat protoplast TaMLO 28.5% 17 Tobacco protoplast and leaf NbPDS, GFP 37.7-38.5% in protoplast; 2.1- 14, 15, 4.8% in leaf agroinfiltration 18 Sorghum immature embryos GFP 28% 15 DNA insertion Human HEK293T and iPS cells AAVS1, eGFP 2.51% 4, 7 Zebrafish embryos etsrp 33.3% 7 Drosophila preblastoderm embryos Yellow 81.3% 9 C. elegans germ line F14E5,nmy-2, his-72, lin-31 9-32% 19 S. cerevisiae CAN1 100% 13 Mouse Tet1/2, Sox2, Nanog, Oct4, 3’UTR, Mecp2 10-58% 20, 21 Arabidopsis protoplast CYCD3, YF-FP reporter 10.7-42% 14, 22, 23 Tobacco protoplast and leaf NbPDS 9% 14 Rice protoplast OsPDS 6.9% 17 DNA deletion Drosophila preblastoderm embryos Yellow 81.3% 9 Human K562 cells AAVS1 16.9% 4 Xenopus tropicalis e mbryos six3 proximal promoter 50% 12 Cas9n and DNA insertion Human HEK 293FT cells EMX1 0.46% 1 tracrRNA:crRNA duplex 2
Double nicking NHEJ-induced indels Human HEK293FT cells EMX1, DYRK1A, GRIN2B Up to 40% 24 by Cas9n-gRNA Mouse zygotes Mecp2 24 complex NHEJ-mediated Human HEK293FT EMX1 24 ligation DNA insertion Human HUES62, HEK293FT cells EMX1 0.4-9.9% 24 DNA deletion Human HEK293FT cells DYRK1A 24 Transcriptional dCas9 and Transcription E. coli gfp-mut2 and its promoter 100-fold repression 25 control tracrRNA:crRNA repression S. pneumoniae bgaA 14- fold repression 25 duplex dCas9 and gRNA Transcription E. coli mRFP, sfGFP, lacZ, lacI, crp , cya 300-fold repression 26 repression individually*; 1,000-fold repression synergistically** Human HEK293 cells EGFP 63% repression 26 S. cerevisiae rTA -binding site of TetON-Venus reporter, 115-fold repression 27 CD71, CXCR4, AP1 enhancer, SP1 enhancer dCas9-effector dCas9-ormega E. coli lacZ, gfp-mut2 23-fold activation 25 and chimera-mediated tracrRNA:crRNA activation duplex dCas9-effector dCas9-VP64 Human HEK293 cells REX1, OCT4, SOX2, NANOG, tdTomato 23-fold activation 27, 28 and gRNA chimera-mediated promoter, Gal4 UAS-GFP reporter individually*; activation 30-fold activation synergistically** dCas9-KRAB Human HEK293 cells EGFP, CD71, CXCR4, AP1 enhancer and 15-fold repression 27 chimera-mediated SP1 enhancer repression dCas9-Mxi1 S. cerevisiae TEF1-GFP 53-fold repression 27 chimera-mediated repression dCas9 and gRNA-MS2 chimera- Human HEK 293T cells REX1 Up to 10-fold activation 28 gRNA-aptamer mediated activation synergistically** Individually*: the efficiency was produced by an individual gRNA. Synergistically**: the efficiency was produced by cooperative works of multiple gRNAs. 3
References: 1. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819-823. 2. Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, Wan J, Gu H, Qu LJ. 2013. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 23 :1233–1236. 3. Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31: 233-239. 4. Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. 2013. RNA-guided human genome engineering via Cas9. Science 339: 823-826. 5. Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. 2013. RNA-programmed genome editing in human cells. Elife 2: e00471. 6. Cho SW, Kim S, Kim JM, Kim JS. 2013. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31: 230-232. 7. Chang N, Sun C, Gao L, Zhu D, Xu X, Zhu X, Xiong JW, Xi JJ. 2013. Genome editing with RNA-guided Cas9 nuclease in Zebrafish embryos. Cell Res. 23: 465-472. 8. Hwang WY, Fu YF, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK. 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31: 227-229. 9. Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O'Connor-Giles KM. 2013. Genome Engineering of Drosophila with the CRISPR RNA-Guided Cas9 Nuclease. Genetics 194: 1029-1035. 10. Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA. 2013. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10: 741- 743. 11. Waaijers S, Portegijs V, Kerver J, Lemmens BB, Tijsterman M, van den Heuvel S, Boxem M. 2013. CRISPR/Cas9-Targeted Mutagenesis in Caenorhabditis elegans . Genetics 195: 1187-1191. 12. Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, Grainger RM. 2013. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis . Genesis 51: 835-843. 13. Dicarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41: 4336-4343. 4
14. Li JF, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J. 2013. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31: 688-691. 15. Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP. 2013. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 41: e188. 16. Xie K, Yang Y. 2013. RNA-guided Genome Editing in Plants Using A CRISPR-Cas System. Mol. Plant 6: 1975-1983. 17. Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu JL, Gao C. 2013. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31: 686-688. 18. Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S. 2013. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31: 691-693. 19. Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028- 1034. 20. Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. 2013. One-Step Generation of Mice Carrying Reporter and Conditional Alleles by CRISPR/Cas-Mediated Genome Engineering. Cell 154: 1370-1379. 21. Wang HY, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. 2013. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas- Mediated Genome Engineering. Cell 153: 910-918. 22. Mao Y, Zhang H, Xu N, Zhang B, Gou F, Zhu JK. 2013. Application of the CRISPR-Cas System for Efficient Genome Engineering in Plants. Mol. Plant 6: 2008-2011. 23. Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, Cao F, Zhu S, Zhang F, Mao Y, Zhu JK. 2013. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23: 1229-1232. 24. Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. 2013. Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell 154: 1380-1389. 25. Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. 2013. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 41: 7429-7437. 5
Recommend
More recommend