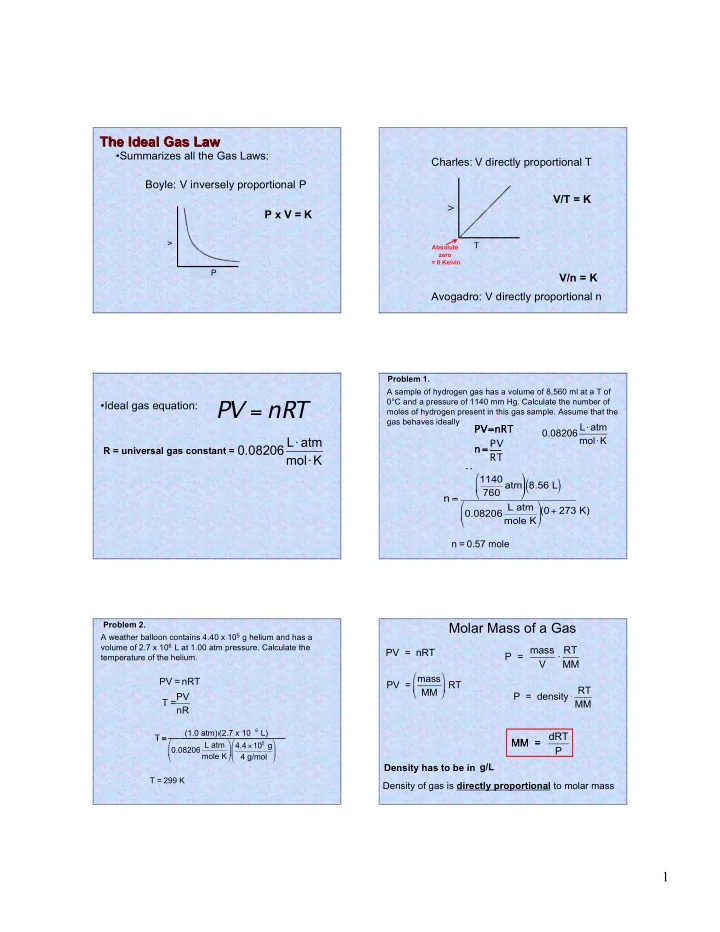

The Ideal Gas Law The Ideal Gas Law •Summarizes all the Gas Laws: Charles: V directly proportional T Boyle: V inversely proportional P V/T = K V P x V = K T v Absolute zero = 0 Kelvin P V/n = K Avogadro: V directly proportional n Problem 1. A sample of hydrogen gas has a volume of 8,560 ml at a T of PV = nRT 0°C and a pressure of 1140 mm Hg. Calculate the number of •Ideal gas equation: moles of hydrogen present in this gas sample. Assume that the gas behaves ideally 0.08206 L � atm PV=nRT PV=nRT PV= 0.08206 L � atm mol � K n = PV n = R = universal gas constant = RT mol � K RT � 1140 � � 760 atm � 8.56 L ( ) � � � � � � � n = = � � 0.08206 L atm � (0 + 273 K) � � � mole K � � � n = 0.57 mole Problem 2. Molar Mass of a Gas A weather balloon contains 4.40 x 10 5 g helium and has a molar mass volume of 2.7 x 10 6 L at 1.00 atm pressure. Calculate the mass mass � RT PV = nRT P = temperature of the helium. � V MM � � � = mass PV = nRT PV = RT RT � � � � RT MM � � � = PV P = density � MM T = nR 6 L) (1.0 atm)(2.7 x 10 (1.0 atm)(2.7 x 10 MM = dRT T = � T = = MM = � � � 0.08206 L atm � 4.4 � 10 5 g � � � P � � � � mole K 4 g/mol � � � � � Density has to be in g/L T = 299 K Density of gas is directly proportional to molar mass 1
Problem 4: An unknown gas has a density of 0.488 g/L at Problem 3: 0.355 atm and 100°C. What is the molar mass of the gas? Arrange the following gases in order of increasing density at 25°C: Ne, Xe, Kr, Ar, H 2 ) 0 . 08206 Latm ) � � � � � 0 . 488g/L � 373K ( ( ) � � dRT molK molK � � � � � � MM = = P 0.355 atm H 2 H 2 < Ne H 2 < Ne < Ar< Kr< Xe H 2 < Ne < Ar H 2 < Ne < Ar< Kr ) = 42 . 7 g/mol II. Gas Stoichiometry Problem 5: Calculate the density (in g/L) of Ar at 32°C and 342 mm Hg. STP: 0 °C = 273 K 1 atm M= dRT When at STP: Calculate the volume of oxygen that gas can be produced by P the complete decomposition of 10.5 g potassium chlorate 40 g � 342 � � �� � � � (MM = 122.6 g/mol) at STP . � � � � d = MP = 0 . 719 g 2 KClO 3 → KCl + O 2 2 3 mol mol mol � 760 760 � � � � � � � � � d = d = = RT 0 . 08206 Latm L � � � � � � � � � � � � � � � � � 1 mol KClO 3 3 mol O 2 22.4L � 32 + 273 ( ) 10.5 g KClO 3 � = 2.88 L � � � � � � � � � � � � � 3 molK 122.6 gKClO 3 2 mol KClO 3 1 mol O 2 � � � � � � � � � � � � � � � Mass Moles Volume Moles O 2 KClO 3 KClO 3 O 2 C 3 H 8 (g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (g) Not at STP: What volume of nitrogen gas is produced at 27°C and 755 If 3.75 L of carbon dioxide gas are produced at STP, what volume of mm Hg if 1.24 g of NI 3 decomposes? (MM = 395 g/mol) oxygen gas is consumed? 2 3 NI 3 → N 2 + I 2 � � � � � � � � � � � � 1 mol CO 2 � 5 mol O 2 � 22.4 L O 2 3.75 L CO 2 � = 6.25 L O 2 � � � � � � � � � 2 � 1 mol NI 3 � 1 mol N 2 � � � � � 22.4 L CO 2 3 mol CO 2 1 mol O 2 � � � � � � � � � � � � 1.24 g NI 1.24 g NI � = 0.00157 mole N 2 � � � � 3 3 395 g NI 3 2 mol NI 3 � � � � � � Moles Moles V CO 2 V O 2 Moles Volume CO 2 O 2 Mass NI 3 Moles N 2 NI 3 N 2 PV = nRT Convert T to K V = nRT (0.00157)(0.08206)(300) (0.00157)(0. )(0.08206)( )(300) 0.0389 L = P 755 Convert P into atm 760 2
C 3 H 8 (g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (g) b) If the reaction begins with 2.75 L of propane ( C 3 H 8 ) gas at 50°C and 752 torr with excess oxygen, what mass of water vapor can be produced? PV = nRT � 752 � � � 2.75 ( ) � � 760 = PV � � � � � n = = 0.1027 mol C 3 H 8 RT = ( ) ) 50 + 273 ( ) 0.08206 � � � 4 mol H 2 O � 18 g H 2 O � � � � � � = 7.39 g H 2 O 0.1027 mol C 3 H 8 � � � � � � 8 1 mol C 3 H 1 mol H 2 O � � � � � � � � Moles mass mol C 3 H 8 H 2 O H 2 O 3
Recommend
More recommend