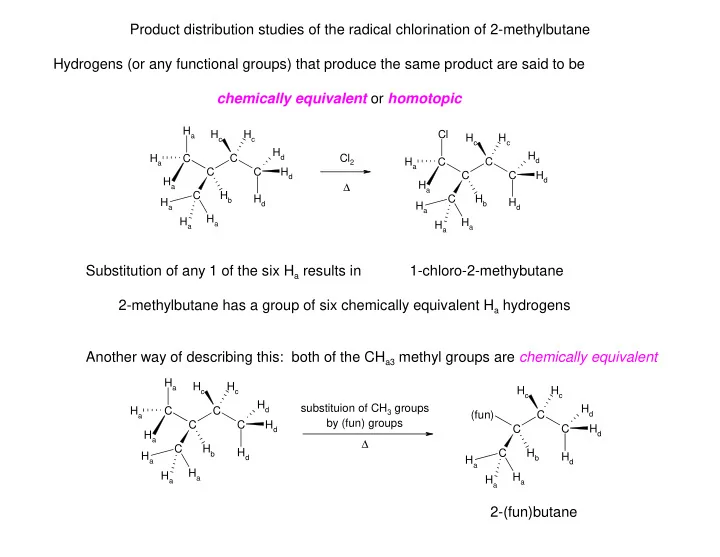

Product distribution studies of the radical chlorination of 2-methylbutane Hydrogens (or any functional groups) that produce the same product are said to be chemically equivalent or homotopic H a H c H c Cl H c H c H d H d H a C C Cl 2 H a C C C C H d C C H d H a ∆ H a C H b H d C H b H a H d H a H a H a H a H a Substitution of any 1 of the six H a results in 1-chloro-2-methybutane 2-methylbutane has a group of six chemically equivalent H a hydrogens Another way of describing this: both of the CH a3 methyl groups are chemically equivalent H a H c H c H c H c H d substituion of CH 3 groups H d H a C C (fun) C by (fun) groups C C H d C C H d H a ∆ C H b H d C H b H a H d H a H a H a H a H a 2-(fun)butane
Notice that replacing one or the other methyl groups with (fun) actually generates enantiomers H a H c H c H c H c H d substituion of CH 3 groups H d H a C C (fun) C by (fun) groups C C H d C C H d H a ∆ C H b H d C H b H a H d H a H a H a H a H a ( R )-2-(fun)butane H a H c H c H a H c H c H d substituion of CH 3 groups H d H a C C C H a C by (fun) groups C C H d C C H d H a H a ∆ C H b H d (fun) H b H a H d H a H a ( S )-2-(fun)butane Because these two methyl groups provide stereoisomers upon substitution, they are said to be stereotopic functional groups . As their substitution leads to enantiomers, they specifically are enantiotopic methyl groups.
H a H c H c H a H c H c H d H a C C Cl 2 H d C H a C C C H d H a C C H d ∆ H a C H b H d H a C Cl H d H a H a H a H a H a Substitution of the H b results in 2-chloro-2-methylbutane 2-methylbutane has a group of one chemically equivalent H b hydrogen The H a ’s and H b are not chemically equivalent Chemically nonequivalent functional groups are heterotopic functional groups Cl H c H c H d H a C C C C H d H a C H b H d H a H a H a 1-chloro-2-methylbutane
H a H c H c H a H a Cl H c H c Cl H d H a C C Cl 2 H d H d H a C C H a C C C C H d C H d C C C H d H a ∆ H a H a C H b H d C H b H a C H b H d H d H a H a H a H a H a H a H a H a ( R )-2-chloro-3-methylbutane or ( S )-2-chloro-3-methylbutane Substitution of an H c results in (a racemate) 2-methylbutane has a group of two chemically equivalent H c hydrogens The H c ’s generate stereoisomers upon substititution These stereoisomers are enantiomers Functional groups that make stereoisomeric products are stereotopic groups If these stereoisomers are enantiomers, the functional groups are enantiotopic H a H c H c Cl H c H c H d H a C C H d H a C C C C H d C C H d H a H a C Cl H d H a C H b H d H a H a H a H a H a 1-chloro-2-methylbutane 2-chloro-2-methylbutane
H a H a H c H c H c H c H d H d H a C C Cl 2 H a C C C C H d C C H d H a H a ∆ C H b C H b H d Cl H a H a H a H a H a H a Substitution of an H d results in 1-chloro-3-methylbutane 2-methylbutane has a group of three chemically equivalent H d hydrogens The CH a3 methyl groups and the CH d3 methyl group are not chemically equivalent So, 2-methylbutane has four groups of chemically nonequivalent hydrogens Cl H a H c H c H c H c H a H c Cl H d H d H d H a C C H a C C C H a C C C H d C C H d C C H d H a H a H a C H b C Cl H d H d C H b H a H a H d H a H a H a H a H a H a H a rac -2-chloro-3-methylbutane 1-chloro-2-methylbutane 2-chloro-2-methylbutane
What if a substitution resulted in diastereoisomeric products? H a H c H c H a H a H c Cl Cl H c H d C Cl 2 H a C H d H d C H a C C H a C C C H d C C H d C C H d ∆ H a H a H a F H b H d F H b F H b H d H d ( S )-2-fluorobutane (2 S, 3 S )-2-chloro-3-fluorobutane (2 R, 3 S )-2-chloro-3-fluorobutane ( S )-2-fluorobutane has two chemically nonequivalent H c hydrogens The H c ’s generate stereoisomers upon substititution These stereoisomers are diasteriomers Functional groups that make stereoisomeric products are stereotopic groups If these stereoisomers are diasteriomers, the functional groups are diastereotopic So, ( S )-2-fluorobutane has five chemically nonequivaent groups of hydrogens
Types of groups of hydrogens Chemically equivalent Chemically nonequivalent (homotopic) (heterotopic) generate the same constitutional generate different constitutiona isomer upon substitution isomers upon substitution generate enantiomers upon generate diastereomers upon substitution (enantiotopic) substitution (diastereotopic)
Recommend
More recommend