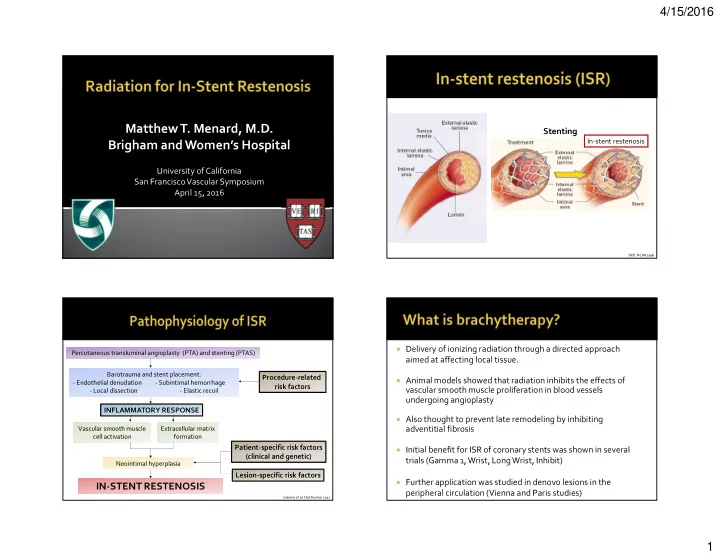

4/15/2016 Matthew T. Menard, M.D. Stenting In-stent restenosis Brigham and Women’s Hospital University of California San Francisco Vascular Symposium April 15, 2016 Bittl. NEJM 1996 � Delivery of ionizing radiation through a directed approach Percutaneous transluminal angioplasty (PTA) and stenting (PTAS) aimed at affecting local tissue. Barotrauma and stent placement: Procedure-related � Animal models showed that radiation inhibits the effects of - Endothelial denudation - Subintimal hemorrhage risk factors vascular smooth muscle proliferation in blood vessels - Local dissection - Elastic recoil undergoing angioplasty INFLAMMATORY RESPONSE � Also thought to prevent late remodeling by inhibiting Vascular smooth muscle Extracellular matrix adventitial fibrosis cell activation formation Patient-specific risk factors � Initial benefit for ISR of coronary stents was shown in several (clinical and genetic) trials (Gamma 1, Wrist, Long Wrist, Inhibit) Neointimal hyperplasia Lesion-specific risk factors � Further application was studied in denovo lesions in the IN-STENT RESTENOSIS peripheral circulation (Vienna and Paris studies) Jukema et al. Nat Review 2012 1
4/15/2016 � Clinical use of radioactive sources to deliver � Radioactive isotopes are made with neutron highly therapeutic and palliative radiation bombardment of stable elements in a reactor therapy to a range of targets or accelerator ▪ gynecological, urological, pulmonary, head and neck, � Large unstable nucleus yearning for peace gastrointestinal, sarcoma, vascular, dermatological, � As nucleus decays emanations occur – endocrine disease � Photons: conservation of mass, energy � Extrinsic electron displaces inner shell electron � Alpha, beta, gamma, neutrinos, bosons, … � Outer shell electron replaces displaced electron � Energy difference between shells is ejected as chargeless wave/particle (conservation of energy) 6 7 102 patients with either new or � EVBT: intraluminal delivery of radiation � restenotic femoropopliteal lesions. Randomized to angioplasty and Gamma � brachytherapy, or angioplasty alone. Tissue No stenting in this trial Stent � growth 6 month restenosis rate: � � 30% angioplasty and brachytherapy vs γ -emitter ( 192 Iridium) � 57% for the angioplasty alone group. Brachytherapy delayed restenosis � recurrence: In-stent restenosis � 17.5 months brachytherapy group vs. � attenuation of collagen synthesis � suppression of monocyte/macrophage activity � 7.4 months in the angioplasty alone � decrement or delay of smooth muscle cell group proliferation � approved by FDA for treatment of ISR in 2000 Radiology 2006 240(3) 878-844 2
4/15/2016 EDGE RESTENOSIS � Restenosis adjacent to the proximal and distal edges of the implanted stent (“edge effect” or “candy wrapper” phenomenon) � Causes: 1. Radioactive dose fall-off at the stent edges 2. Failure of stent to treat the barotraumatized margins Distal Proximal barotrauma barotrauma 11 Source length Primary patency Treatment type Reference 6 months 1 year Repeat balloon angioplasty 27% -- Dick et al. Radiology 2008 Target localization (ISR lesion, angioplasty) Cutting balloon angioplasty 35% -- Dick et al. Radiology 2008 50% 0% Karthik et al. EJVES 2007 Cryoplasty -- 28% Schmieder et al. JVS 2010 Directional atherectomy -- 54% Zeller et al. JACC 2010 distal proximal Excimer laser and “safety margin” “safety margin” -- 48% Laird et al. Card Cath Int 2012 stent-graft Key features: PTA, laser, or excisional 55% 47.6% Yeo et al. Card Cath Int 2011 1. Higher radiation dose (20 gray) atherectomy 2. 2 cm“safety margins” of radiation coverage proximal and distal to (70%) -- Vienna 4 (2001) angioplastied/stented area PTA+EVBT (67%) (57%) Vienna 5 (2005) 95.2% 79.8% Leipzig 2012 Customized treatment depth: 0.5mm + radius of largest PTA balloon 3. 3
4/15/2016 Retrospective, single-center review of 43 cases of EVBT for lower extremity ISR � at Brigham and Women’s Hospital between 2004-2012 Mean age (years) (± standard deviation) 67.0±11.4 Indication for original stents All patients were evaluated by radiation oncologist and consented for EVBT � Female gender, N (%) 16 (38.1%) Claudication 34 (81%) ahead of time Smoking Critical limb ischemia 8 (19%) Former 19 (50.0%) Stent location Aspirin and clopidogrel indefinitely Current 6 (15.8%) Common iliac artery 3 (7%) � Diabetes 20 (47.6%) External iliac artery 6 (14%) Hypertension 36 (85.7%) Superficial femoral artery 26 (62%) Stents undergo duplex ultrasound surveillance for recurrent ISR at 1, 3, 6, 9, 12, � Hypercholesterolemia 31 (73.8%) Popliteal artery 2 (45%) and 18 months and then yearly Combined SFA and 5 (12%) Chronic kidney disease (serum creatinine ≥ 7 (16.8%) popliteal segments 2mg/dL) Primary endpoint: stent patency (primary, primary-assisted, and secondary) at 1 � End stage renal disease 2 (4.8%) and 2 years � Stent patency: freedom from ≥ 50% recurrent stenosis by duplex ultrasound Calibrated SFA in-stent dummy restenosis strand for before PTA EVBT planning SFA in-stent restenosis after PTA 4
4/15/2016 Femoropopliteal ISR lesions, N (%) 33 (76.7%) � Technical success: 42/43 (97.6%) Additional stenting at time of EVBT 10 (31.3%) Location of ISR: 26 (60.5%) Mean EVBT treated length, cm (± SD) 23.6±13.1 Superficial femoral artery (SFA) � Follow-up time: 706.3±543.7 days Popliteal artery 2 (4.7%) Maximum PTA balloon diameter (in mm) Combined SFA and popliteal arteries 5 (11.9%) � Symptom status: 2 (6.3%) 4 4.5 1 (3.1%) Indication for EVBT: 5 17 (53.1%) Claudication 16 (50.0%) � Claudicants: 6 Critical stenosis on duplex 12 (37.5%) 13 (40.6%) Critical limb ischemia ▪ resolved in 18/20 (85%) 3 (9.4%) Mean total radiation time, minutes (± SD) 16.6 ±9.8 Tibial runoff at time of EVBT (number of At least 1 re-intervention for ISR prior to EVBT 11 (34.4%) ▪ Improved and then recurred in 2/20 vessels): 5 (19.2%) Mean ABI (± SD): 1 6 (23.1%) Pre-EVBT 0.76±0.22 � Mean ABI change: +0.14±0.23 (range -0.21- 2 Post-EVBT 15 (57.7%) 0.91±0.18 3 0.84) � Recurrent ISR 50-99% stenosis after EVBT: 8/42 (19.1%) � Mean time to recurrent ISR: 505±348 days 6 months � In-stent recurrence: 4/8 Time after 1 year 2 years (180 EVBT (365 days) (730 days) � In-segment recurrence: 4/8 days) Primary 87.5% 75.2% 63.7% patency (NAR=32) (NAR=23) (NAR=11) � Early thrombotic occlusion: 2/42(4.7%) Primary 92.1% 89.1% 80.6% � Time to occlusion: 1 day, 26 days assisted (NAR=32) (NAR=29) (NAR=15) patency � Late thrombotic occlusion: 5/42 (11.9%) Secondary 92.1% 89.1% 85.6% � Mean time to recurrent ISR: 708 ± 368 days patency (NAR=33) (NAR=29) (NAR=16) � Death: 1 (possible acute coronary syndrome) 5
4/15/2016 Primary patency Treatment type References 6 months 1 year 2 years Balloon angioplasty 27% -- -- Dick et al. Radiology 2008 Time after 6 months 1 year 2 years Cutting balloon 35% -- -- Dick et al. Radiology 2008 EVBT (180 days) (365 days) (730 days) angioplasty Primary 86.6% 78.5% 66.8% 50% 0% -- Karthik et al. EJVES 2007 Cryoplasty patency (NAR=22) (NAR=17) (NAR=7) -- 28% -- Schmieder et al. JVS 2010 Primary Directional atherectomy -- 54% Zeller et al. JACC 2010 89.7% 85.4% 76.9% assisted Excimer laser and (NAR=23) (NAR=19) (NAR=8) -- 48% -- Laird et al. Card Cath Int 2012 patency stent-graft Secondary 66.8% 85.4% 85.4% PTA, laser, or excisional 55% 47.6% Yeo et al. Card Cath Int 2011 patency (NAR=7) (NAR=19) (NAR=9) atherectomy (70%) -- -- Vienna 4 (2001) PTA+EVBT (67%) (57%) -- Vienna 5 (2005) 95.2% 79.8% -- Leipzig 2012 PTA+EVBT 86.6% 78.5% 66.8% Current study � Retrospective review of consecutive patients who underwent brachytherapy for angiographically proven instent � All patients were pretreated with Aspirin 325 mg, and Plavix restenosis, thrombosis, or occlusion load of 300-600mg pre or immediately post procedure. � Data collected between December 2003 to February 2010 at Brigham and Women’s Hospital cardiac catheterization � Intra-arterial heparin with a goal ACT (activated clotting laboratory. time) of >250 � Thirty two patients were identified including 42 lower extremities � Performed by 3 operators � Decisions regarding provisional brachytherapy were made � Lesions included 31 SFA stents, 10 iliac stents, and 1 popliteal prior to cath, and were based on a combination of stent. symptoms, ABIs, and ultrasound findings. � Patient follow-up duration has been 5 years (and ongoing) 6
4/15/2016 Characteristic N Age (years, range) 66 (52-84) Gender (Male) 18/32(56%) CAD 24/32(75%) HTN 31/32(97%) CRI 4/32(12.5%) Smoker 26/32(81%) DM 14/32 (44%) Statin 29/32 (91%) ACEi 22/32 (69%) Index procedural Indication N Brachytherapy Indication N Claudication 39/42 (93%) Claudication 40/42 (95%) Critical limb ischemia 3/42 (7%) Critical limb ischemia 1/42 (2.5%) Index intervention N Ultrasound (high grade stenosis, no 1/42 (2.5%) symptoms) Iliac 10/42 (24%) SFA 32/42 (76%) Mode of index stent failure N Popliteal 1/42 (2%) Restenosis 31/42 (74%) Occlusion 9/42 (21%) Index Lesion N Thrombosis 1/42 (2.5%) Lesion length (mean, range) 266, 40-480 mm Chronic total occlusion 24/42 (57%) Unknown 1/42 (2.5%) Stenosis 18/42 (43%) 7
Recommend
More recommend