Mathematical models of chemical reactions The Law of Mass Action - PowerPoint PPT Presentation
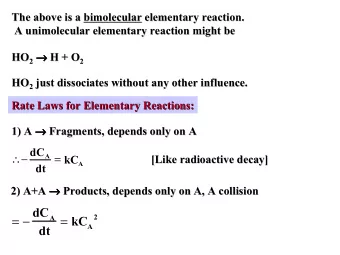
Mathematical models of chemical reactions The Law of Mass Action Chemical A and B react to produce chemical C: k A + B C The rate constant k determines the rate of the reaction. It can be interpreted as the probability that a collision
Mathematical models of chemical reactions
The Law of Mass Action Chemical A and B react to produce chemical C: k A + B → C − The rate constant k determines the rate of the reaction. It can be interpreted as the probability that a collision between the reactants produces the end results. If we model the probability of a collision with the product [A] [B] we get the law of mass action: d [ C ] = k [ A ][ B ] dt
A two way reaction The reverse reaction may also take place: k + − → A + B C ← − k − The production rate is then: d [ C ] = k + [ A ][ B ] − k − [ C ] dt At equilibrium when d [ C ] / dt = 0 we have: k − [ C ] = k + [ A ][ B ] (1)
k If A + B → C is the only reaction involving A and C then − d [ A ] / dt = − d [ C ] / dt so that [ A ] + [ C ] = A 0 (2) Substituting (2) into (1) yields: [ B ] [ C ] = A 0 K eq + [ B ] where K eq = k − / k + . Notice that [ B ] = K eq = [ C ] = A 0 / 2 ⇒ and [ B ] → ∞ = [ C ] → A 0 ⇒
Gibbs free energy Molecules have different chemical potential energy, quantified by Gibbs free energy G = G 0 + RT ln( c ) where c is the concentration of the molecule, T is the temperature, R the gas constant. G 0 is the energy at c = 1M, called the standard free energy .
Gibbs free energy Can be used to compare two states: A − → B Change in free energy after this reaction: ∆ G = G B − G A = ( G 0 B + RT ln( B )) − ( G 0 A + RT ln( A )) = ( G 0 B − G 0 A ) + ( RT ln( B ) − RT ln( A )) = ∆ G 0 + RT ln( B / A ) If ∆ G < 0, i.e. there is less free energy after the reaction, then B is the preferred stated.
Gibbs free energy at equilibrium At equilibrium neither states are favoured and ∆ G = 0: ∆ G = ∆ G 0 + RT ln( B / A ) = 0 Given G 0 , the concentrations at equilibrium must satisfy: ln( B eq / A eq ) = − ∆ G 0 / RT or B eq = e − ∆ G 0 / RT A eq
Gibbs free energy and rate constants The reaction k + − → A B ← − k − is governed by d [ A ] = k − [ B ] − k + [ A ] dt and at equilibrium d [ A ] dt = 0, so k − [ B ] − k + [ A ] = 0 , or , A / B = k − / k + = K eq Comparing with the Gibbs free energy we find: K eq = e ∆ G 0 / RT Note: ∆ G 0 < 0 = ⇒ K eq < 1 = ⇒ B eq > A eq
Gibbs free energy with several reactants The reaction α A + β B − → γ C + δ D has the following change in free energy: ∆ G = γ G C + δ G D − α G A − β G B = γ G 0 C + δ G 0 D − α G 0 A − β G 0 B + γ RT ln([ C ]) + δ RT ln([ D ]) − α RT ln([ A ]) − β RT ln([ B ]) = ∆ G 0 + RT ln([ C ] γ [ D ] δ [ A ] α [ B ] β ) At equilibrium with ∆ G = 0: eq [ B ] β ∆ G 0 = RT ln( [ A ] α eq ) [ C ] γ eq [ D ] δ eq
Detailed balance Consider the cyclic reaction: In equilibrium all states must have the same energy: G A = G B = G C All transitions must be in equilibrium: k 1 [ B ] = k − 1 [ A ] , k 2 [ A ] = k − 2 [ C ] , k 3 [ C ] = k − 3 [ B ] Which yields: k 1 [ B ] · k 2 [ A ] · k 3 [ C ] = k − 1 [ A ] · k − 2 [ C ] · k − 3 [ B ]
Detailed balance cont. k 1 [ B ] · k 2 [ A ] · k 3 [ C ] = k − 1 [ A ] · k − 2 [ C ] · k − 3 [ B ] so k 1 k 2 k 3 = k − 1 k − 2 k − 3 This last condition is independent of the actual concentrations and must hold in general. Thus only 5 free parameters in the reaction.
Enzyme Kinetics Characteristics of enzymes: Made of proteins Acts as catalysts for biochemical reactions Speeds up reactions by a factor > 10 7 Highly specific Often part of a complex regulation system
Reaction model of enzymatic reaction k 1 k 2 − → S + E k − 1 C → P + E − ← − with S: Substrate E: Enzyme C: Complex P: Product
Mathematical model of enzymatic reaction Applying the law of mass action to each compound yields: d [ S ] = k − 1 [ C ] − k 1 [ S ][ E ] + J S dt d [ E ] = ( k − 1 + k 2 )[ C ] − k 1 [ S ][ E ] dt d [ C ] = k 1 [ S ][ E ] − ( k 2 + k − 1 )[ C ] dt d [ P ] = k 2 [ C ] − J P dt Here we also supply the substrate at rate J S and the product is removed at rate J P .
Equilibrium Note that In equilibrium d [ S ] / dt = d [ E ] / dt = d [ C ] / dt = d [ P ] / dt = 0 it follows that that J S = J P . Production rate: J = J P = k 2 [ C ]
In equilibrium we have d [ E ] = 0 dt that is ( k − 1 + k 2 )[ C ] = k 1 [ S ][ E ] Since the amount of enzyme is constant we have [ E ] = E 0 − [ C ] This yields E 0 [ S ] [ C ] = K m + [ S ] with K m = k − 1 + k 2 and E 0 is the total enzyme concentration. k 1 Production rate: d [ P ] [ S ] = k 2 [ C ] = V max K m +[ S ] , where V max = k 2 E 0 . dt
Cooperativity, 1.4.4 k 1 k 2 − → S + E k − 1 C 1 → E + P − ← − k 3 k 4 − → S + C 1 → C 1 + P k − 3 C 2 − ← − with S: Substrate E: Enzyme C1: Complex with one S C1: Complex with two S P: Product
Mathematical model of cooperativ reaction Applying the law of mass action to each compound yields: ds = − k 1 se + k − 1 c 1 − k 3 sc 1 + k − 3 c 2 dt dc 1 = k 1 se − ( k − 1 + k 2 ) c 1 − k 3 sc 1 + ( k 4 + k − 3 ) c 2 dt dc 2 = k 3 sc 1 − ( k 4 + k − 3 ) c 2 dt
Equilibrium Set dc 1 dt = dc 2 dt = 0, and use e 0 = e + c 1 + c 2 , K 2 e 0 s = c 1 K 1 K 2 + K 2 s + s 2 e 0 s 2 c 2 = K 1 K 2 + K 2 s + s 2 where K 1 = k − 1 + k 2 , K 2 = k 4 + k − 3 k 1 k 3 Reaction speed: V = k 2 c 1 + k 4 c 2 = ( k 2 K 2 + k 4 s ) e 0 s K 1 K 2 + K 2 s + s 2
Case 1: No cooperation The binding sites operate independently, with the same rates k + and k − . k 1 , k − 3 and k 4 are associated with events that can happen in two ways, thus: k 1 = 2 k 3 = 2 k + k − 3 = 2 k − 1 = 2 k − k 4 = 2 k 2 So: K 1 = k − 1 + k 2 = k − + k 2 = K / 2 k 1 2 k + K 2 = k − 3 + k 4 = 2 k − + 2 k 2 = 2 K k 3 k + where K = k − + k 2 k +
Which gives this reaction speed: ( k 2 K 2 + k 4 s ) e 0 s V = K 1 K 2 + K 2 s + s 2 (2 k 2 K + 2 k 2 s ) e 0 s = K 2 + 2 Ks + s 2 2 k 2 ( K + s ) e 0 s = 2 k 2 e 0 s = ( K + s ) 2 ( K + s ) Note that this is the same as the reaction speed for twice the amount of an enzyme with a single binding site.
Case 2: Strong cooperation The first binding is unlikely, but the next is highly likely, i.e. k 1 is small, and k 3 is large. We go to the limit: k 1 → 0 , k 3 → ∞ , k 1 k 3 = const so K 2 → 0 , K 1 → ∞ , K 1 K 2 = const In this case the reaction speed becomes: V = k 4 e 0 s 2 s 2 m + s 2 = V max K 2 K 2 m + s 2 with K 2 m = K 1 K 2 , and V max = k 4 e 0
The Hill equation In general with n binding sites, the reaction rate in the limit will be: s n V = V max K n m + s n This model is often used when the intermediate steps are unknown, but cooperativity suspected. The parameters V max , K m and n are usually determined experimentally.
Recommend
More recommend
Explore More Topics
Stay informed with curated content and fresh updates.