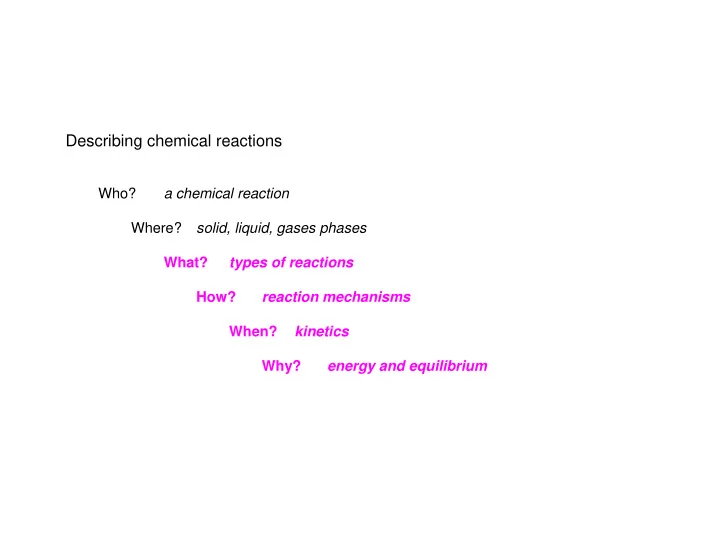

Describing chemical reactions a chemical reaction Who? solid, liquid, gases phases Where? What? types of reactions How? reaction mechanisms When? kinetics Why? energy and equilibrium
types of reactions What is happening in a chemical reaction? 1) Addition A + B 6 6 6 6 C H H H H cat. OH H OH + H H H H H 2) Elimination A 6 6 B + C 6 6 H H H H OH cat. H OH H + H H H H
3) Substitution A–B + C–D 6 6 6 A–C + B–D 6 H H H H OH H Br + H OH + H Br H H H H H 4) Rearrangement A 6 6 B 6 6 O O cat.
Why is a chemical reaction happening? energy & equilibrium the change in enthalpy ∆ H of a reaction • a reaction can be endothermic ( ∆ H is positive) or exothermic ( ∆ H is negative) ∆ H of a reaction can be measured using a calorimeter • ∆ H of a reaction can be calculated using bond dissociation energies • • Consider the addition reaction: Br + H Br C 3 H 6 + HBr = C 3 H 7 Br
Br + H Br
The change in entropy ∆ S of a reaction and Gibbs free energy ∆ G = ∆ H - T( ∆ S) if ∆ G = + the reaction is not spontaneous • if ∆ G = - the reaction is spontaneous • generally speaking, for most organic reactions ∆ S is small compared to ∆ H •
Gibbs free energy and reaction equilibrium ∆ G = -RTlnK eq or ∆ G = e - ∆ G/RT What’s the K eq of Br + H Br ?
Knowing that K eq = 8 x 10 7 mol -1 , ∆ G = -RTlnK eq = -RTln(8 x 10 7 mol -1 ) = -(1.99 x 10 -3 kcal E K -1 )(298 E K)ln(8 x 10 7 mol -1 ) ∆ G = -11 kcal mol -1 Recall that we calculated ∆ H = -15 kcal mol -1 so with ∆ G = ∆ H - T( ∆ S), ∆ S = (11 kcal mol -1 -15 kcal mol -1 )(298 E K) -1 = -1.3 x 10 -2 kcal E K -1
How is a chemical reaction happening? reaction mechanisms A reaction mechanism is the process by which chemical bonds are broken and formed in sequence so as to convert starting reagents into the observed products. There are three ways to break and to make covalent bonds: homolytic bond cleavage / homogenic bond formation . . H Br Br H (radicals) . . H Br H Br reactions involving radicals and sequential homoytic bond cleavage are called radical reactions .
heterolytic bond cleavage / heterogenic bond formation : H + Br- H Br (ions) : H + Br- H Br reactions involving ions and sequential heterolytic bond cleavage are called polar or ionic reactions .
simultaneous bond cleavage / bond formation Y A A Y z E E z reactions that occur in one step without intermediates of any kind are called nonpolar concerted reactions .
Ionic or polar mechanisms Four characteristic patterns: 1. Nucleophilic attack
Ionic or polar mechanisms Four characteristic patterns: 2. Loss of a leaving group
Ionic or polar mechanisms Four characteristic patterns: 3. Proton transfer
Ionic or polar mechanisms Four characteristic patterns: 4. Rearrangement
Recommend
More recommend