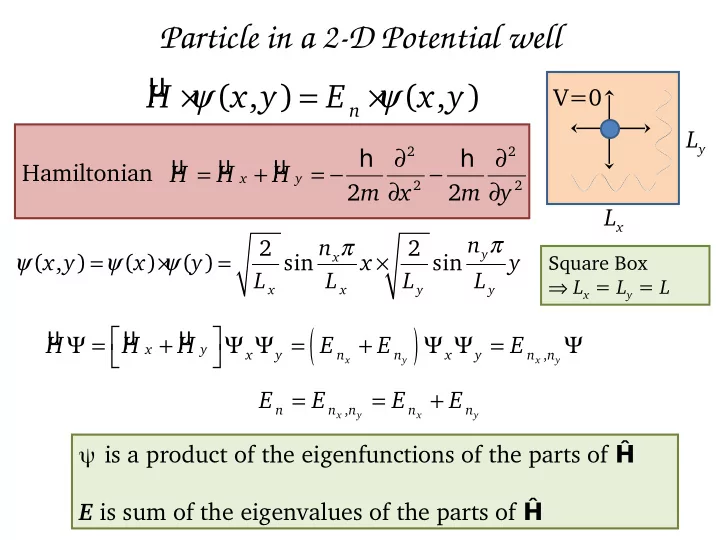

Particle in a 2-D Potential well µ × ψ = × ψ ( , ) ( , ) H x y E x y V=0 n L y ∂ ∂ h h 2 2 µ µ µ = + = − − Hamiltonian H H H x y ∂ ∂ 2 2 2 m x 2 m y L x π π n 2 n 2 ψ = ψ × ψ = × y x ( , ) x y ( ) x ( ) y sin x sin y Square Box L L L L ⇒ L x = L y = L x x y y ( ) µ µ µ Ψ = + Ψ Ψ = + Ψ Ψ = Ψ H H H E E E x y , x y n n x y n n x y x y = = + E E E E , n n n n n x y x y ψ is a product of the eigenfunctions of the parts of Ĥ E is sum of the eigenvalues of the parts of Ĥ
Degeneracy is manifestation of symmetry Energy
2-D Potential Well - Wavefunctions Number of nodes = n i -1 What Quantum Numbers in x and y do this wavefunction correspond to?
Expectation Values and Probability ∂ ∫ ∫ ∗ = ψ ∗ × − × × ψ = ψ × × × ψ h p i ÷ dx x x dx ∂ x x π π 2 2 n n L ∫ π ∂ π = × × × 2 n 2 n sin x x sin x dx L ∫ = − × × × h i sin x sin x dx L L L L 0 ∂ L L x L L 0 π 2 n ∫ L − π π π h = × × 2 2 i n n n x sin x dx ∫ L = × × sin x cos x dx L L 0 2 L L L 0 Probability of finding the particle in a given small interval (x 1 ,x 2 ) π 2 n x x x ∫ ∫ = Ψ Ψ = = 2 * 2 2 P x x ( , ) dx Sin ...( solve ) ÷ 1 2 L L x x 1 1 π 2 n x ≈ ∆ ∆ = − 2 ( , ) . if ( ) is small P x x Sin ÷ x x x x 1 2 2 1 L L
Particle in a 2-D Well: Energies V=0 2 n 2 2 h n y = + E x ÷ , ÷ n , n 2 2 8 m L L x y x y = n n , 1,2,3,4,.... x y V=0 L y L x Square Box ⇒ L x = L y = L 2D Well: 2 Quantum Numbers are required to describe system
Particle in a 3D-Box:Wavefunctions 3-D Box: 3 Quantum Numbers Cubic Box: Wavefunctions
Importance in Chemistry/Spectroscopy • Electronic spectra of conjugated molecules (loose p-electrons): 1D-PIB Region II Region I Region III V=0 V= ∞ V= ∞ X=0 X X=L → Spectroscopy of PIB in D n 1 : n i f Increasing length of the box 2 h ν = ∆ = − = − 2 2 h E E E ( n n ) 8 (i.e. the conjugation length) f i f i 2 mL reduces the energy gaps and hence lower energy photons Longer the box smaller the energy of hv , are absorbed or emitted
Nanoscience: Quantum Confinement Quantum Dots – Particle (excitons) in a Sphere! - + - + - + CB CB CB VB VB VB Quantum Dots have a huge application In chemistry, biology, and materials science For photoemission imaging purpose, Band gap changes due to confinement, As well as light harvesting/energy science and so will the color of emitted light
CH1037Lecture 5 AC The Hydrogen Atom The Hydrogen Atom 2 m θ 2 sin A Completely Solvable Completely Solvable problem!! problem!! A (kind of rare, in QM!) (kind of rare, in QM!)
What we learnt from solving PIB? Formulate a correct Hamiltonian (energy) Operator H Solve H Ψ =E Ψ (2 nd order PDE) by separation of variable and intelligent trial/guess solutions Impose boundary conditions for eigenfunctions (restriction) and obtain Quantum Numbers Eigenstates or Wavefunctions: Should be “well behaved” - Normalization of Wavefunction Energies of states Quantum Numbers Corresponding to that specify the Probability and Quantum Numbers “state” of the system Average Values
H-Atom: Constructing H=T+V µ 2 ∂ ∂ ∂ h 2 2 2 2 P ( , , ) x y z N µ µ ∑ = + = + = − + + + H KE PE V x y z t ( , , , ) V x y z t ( , , , ) ÷ ∂ ∂ ∂ 2 2 2 2 m 2 m x y z = i 1 i i i i ∂ ∂ ∂ µ = − + + = Q h P i and V x y z t ( , , , ) Potential energy ÷ ∂ ∂ ∂ x y z N 2 ∑ h ∂ ∂ ∂ 2 2 2 ¶ µ = − ∇ + ∇ 2 2 H V = + + → , where ( Laplacian ) ; i particles i i 2 m ∂ ∂ ∂ 2 2 2 x y z = i 1 i i i i r Hydrogenic Atoms: 2-Particle System 1 electron moving around a (massive) central nucleus (+ve) h 2 h 2 · ¶ = − − H ∇ ∇ V 2 2 + − Electron Nucleus Nucleus Electron 2 m 2 m Nucleus Electron
Potential Energy: Coulomb Potential 2 2 h h · ¶ = − − H V ∇ ∇ 2 2 + − Electron Nucleus Nucleus Electron 2 m 2 m Nucleus Electron 2 2 Ze Ze µ = = − − Potential Energy V U r ( ) ~ : ( Coulomb Potential ) πε 4 r r 0 2 Ze ∂ ∂ ∂ ∂ ∂ ∂ h 2 2 2 2 h 2 2 2 2 · = − − − H r + + + + ÷ ÷ r ∂ ∂ ∂ ∂ ∂ ∂ 2 2 2 2 2 2 2 m x y z 2 m x y z N N N N e e e e ( ) ( ) ( ) 2 2 2 = − + − + − where r x x y y z z e N e N e N Ψ = − If Complete Wavefunction for H Atom : TISE becomes Total 2 2 2 h h Ze − − ∇ Ψ ∇ Ψ Ψ Ψ 2 2 E − = , e N Total Total Total Total Total 2 m 2 m r N e Ψ Ψ = + where ( , x y z x , , y , z ) and E E E = , Total e e e N N N Total e N
Reduced Form of TISE for H-Atom: Separation of Variables Movement of electronmuch faster than heavy nucleus : + m x m x = − = x x x x e e N N ⇒ e N CM Separate translational motion relative frame . M + m y m y = − = y y y y e e N N Separate H in terms of CM and electronic coordinates e N CM M Ψ x y z x , , , y , z = Ψ x y z , , •Ψ x , y , z ( , ) ( ) ( ) + m z m z Total e e e N N N e e e e N N N N = − = z z z z e e N N 2 h e N CM − M ∇ Ψ Ψ 2 and = E CM N N N 2 M m m µ = Reduced Mass: e N 2 2 h Ze − M ∇ Ψ Ψ 2 E − = ÷ e e e e µ 2 r Free Particle: movement of The whole atom: You solved it! Relative motion of the electron and With respect to the Nucleus Relative motion of electron wrt nucleus: ∂ ∂ ∂ h 2 2 2 2 2 Ze Ψ − Ψ + Ψ + Ψ − Ψ E ( , , ) x y z ( , , ) x y z ( , , ) x y z ( , , ) x y z = ( , , ) x y z ÷ e e µ ∂ e ∂ e ∂ e e 2 2 2 2 x y z + + 2 2 2 x y z i i i Problem: 2 nd order PDE with 3 variables - can not be separated!
Spherical Polar Coordinates Conversion from Cartesian coordinates Used for spherically symmetric systems
Hamiltonian: Spherical Polar Coordinates Looks can be deceiving! Looks can be deceiving! Solve this PDE need to separate variables r , θ, φ : POSSIBLE
Recommend
More recommend