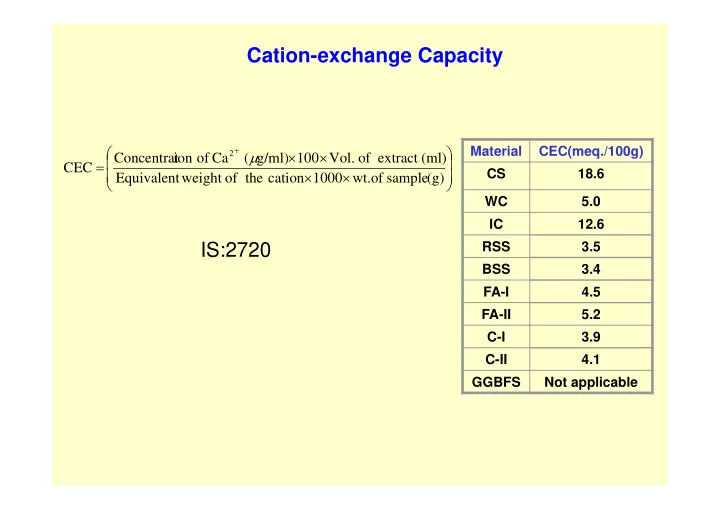

Cation-exchange Capacity Material CEC(meq./100g) 2 Concentrat ion of Ca ( g/ml) 100 Vol. of extract (ml) CEC CS 18.6 Equivalent weight of the cation 1000 wt. of sample (g) WC 5.0 IC 12.6 RSS 3.5 IS:2720 BSS 3.4 FA-I 4.5 FA-II 5.2 C-I 3.9 C-II 4.1 GGBFS Not applicable
Scanning Electron Microscopy (SEM) For obtaining very detailed images at much higher magnifications ~100,000x than is possible with a light microscope. The SEM images the surface structure of bulk specimens (biological, medical, materials sciences and earth sciences) Image is created by using electrons instead of light waves. Images have a greater depth of field and resolution than optical Micrographs. Ideal for fracture surfaces & particulate materials. Energy Dispersive Spectrometer (EDS) allows elemental analysis (Sodium to Uranium, excluding Lanthanides, Actinides & gases down to levels of ~0.1 wt %) with the SEM. X-ray mapping is also possible, which shows the distribution of elements in the material. X-ray line-scans show the concentration variation of elements along a line in the material.
SEM- Working principle • A beam of highly energetic electrons is focused on the sample • Interaction of electrons is transformed into a 3-D image to obtain topographical, morphological, compositional & crystallographic information.
Determination of fabric structure of fine-grained soils Using SEM Compacted sample Cubic specimen Specimen preparation (Challenges): • Removal of pore fluid from the specimen without disturbing its microstructure. • Freeze-drying technique (for swelling/shrinking type of soils) • Air-drying technique (for non swelling/shrinking type of soils) • Specimen should be able to withstand the vacuum inside the microscope. • As illumination is with electrons, specimen should be made to conduct electricity. • Specimen are coated with a very thin layer of Gold or Carbon (a sputter coater). • Gold coating film can absorb X-ray signal generated into the specimen. • For obtaining X-ray spectrum of a non-conducting sample a coating material very transparent to the X-ray (Carbon) must be utilized.
Kaolinite plate stacks Face-Face interaction
Face-Edge & Edge-Edge interactions
Mercury Intrusion Porosimetry (MIP) Geomaterials are composed of wide range of particle sizes and shapes and are porous in nature. A knowledge of pore structure of these materials is important as it can give insight in to both the microstructure and the performance. Rather than measuring the porosity, It becomes more informative if the manner in which volume is distributed With respect to pore size. Inter-connected Closed Passing Dead end
Porosity Porous solids Non-porous solids medium high surface area, pore (Extremely low surface area) volume and dimension Catalysts: Particulates activated sites on porous particle size and surface area support or powder
Shape of Pores Cylindrical Slits Spherical or Interstices Ink Bottle Conical
Pore size classification and parameters Micropores: 0 < d < 2 nm (zeolites, carbons, silica fumes) Mesopores: 2 < d < 50 nm (alumina, polymers, catalysts) Macropores: 50 < d < ...nm (rocks, cements, soils, ...) Bulk, apparent and real density [g/ cc] Percentage porosity [% ] Pore volume/ pore size distribution [pore volume vs pore size] Total pore volume [cc/ g] Average pore size S pecific surface area [m2/ g] Particle size distribution [relative percentage vs particle size]
Characterization schemes Pore size distribution Particle size distribution Mercury porosimetry Bulk density Apparent density Total porosity Pore area distribution Low/high specific surface Gas adsorption Micro/mesopores distribution Micro/mesopores total volume Helium Pycnometry Real density
Mercury Intrusion Porosimetry (MIP) • Mercury intrusion Porosimetry is regarded as a standard measure for macro and meso pore size distributions. • Since this technique is Conceptually much simpler. • Experimentally much faster . • Unique in its ability to evaluate a much wider range of pore sizes than the alternative methods (gas sorption , calorimetry, scanning electron microscopy, thermoporometry). • The technique of mercury Porosimetry is used not only to determine the distribution of pores in various soils but also how it changes for various loading conditions
Mercury Porosimetry concept non wetting wetting • Hg is a non-wetting liquid for many solids • Hg must be forced to penetrate pores • Penetration pressure is related to pore size • Volume of Hg is related to pore volume
Working principle: P = 2.( T .cos θ )/ r ……Washburns Equation Typical MIP characteristic curve Intrusion curve Extrusion curve Volume of mercury Information obtained A the pore size distribution surface area Pressure equivalent pore size A: hysterisis critical pore diameter distribution of total porosity free porosity and trapped porosity
Two systems presenting similar mercury intrusion test results
Recommend
More recommend