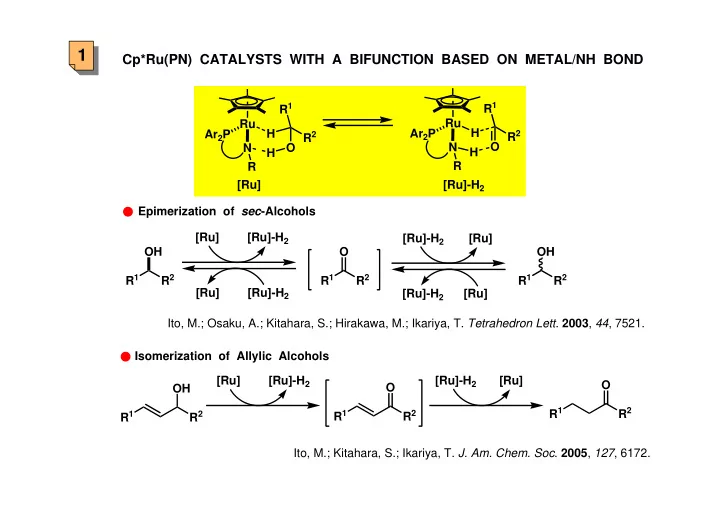

1 Cp*Ru(PN) CATALYSTS WITH A BIFUNCTION BASED ON METAL/NH BOND R 1 R 1 Ru Ru H H Ar 2 P Ar 2 P R 2 R 2 O O N N H H R R [Ru] [Ru]-H 2 Epimerization of sec -Alcohols [Ru] [Ru]-H 2 [Ru]-H 2 [Ru] OH O OH R 1 R 2 R 1 R 2 R 1 R 2 [Ru] [Ru]-H 2 [Ru]-H 2 [Ru] Ito, M.; Osaku, A.; Kitahara, S.; Hirakawa, M.; Ikariya, T. Tetrahedron Lett . 2003 , 44 , 7521. Isomerization of Allylic Alcohols [Ru] [Ru]-H 2 [Ru]-H 2 [Ru] O O OH R 1 R 2 R 1 R 2 R 1 R 2 Ito, M.; Kitahara, S.; Ikariya, T. J. Am. Chem. Soc . 2005 , 127 , 6172.
2 LACTONIZATION OF SYMMETRICAL DIOLS O Ru cat R R O OH OH KO t -C 4 H 9 + OH + 2 2 O acetone R R 30 °C, 1 h TOF >1000 h − 1 S/C = 100, Ru cat:KO t -C 4 H 9 = 1:1 [diol] = 0.1 - 1.0 M O O O O O O O O O O >99% yield >99% >99% >99% >99% Ph 2 P O Ru O O O O N Cl O H 2 O O O O Ru cat Ph Ph >99% >99% 97% 99% >99% The 81st Annual Meeting of Chemical Society of Japan . 2002 , 3G414
3 POSSIBLE MECHANISM FOR LACTONIZATION WITH Cp*Ru CATALYST O Ru cat R R O OH OH KO t -C 4 H 9 + OH + 2 2 O acetone R R O Ar Ru P H Ar OH OH N H H OH O OH base Ar Ru Ru Ru P Ar Ar P NH Cl P NH Ar - HCl OH N Ar Ar H O H OH Ar Ru O P H Ar O N O H H
4 REGIOSELECTIVE LACTONIZATION OF UNSYMMETRICAL 1,4-DIOLS 2-Substituted-1,4-diols O Ru cat 1 R R R 1 1 KO t -C 4 H 9 OH 2 2 2 + O O OH acetone 4 4 4 O 4-Substituted-1,4-diols O Ru cat 1 1 1 KO t -C 4 H 9 OH OH + O OH O acetone 4 4 4 R R R 4-Substituted-triols 1 O O Ru cat OH 1 1 KO t -C 4 H 9 OH O O + 4 acetone n 4 n 4 HO n OH HO (n = 1, 2, 3)
5 REPORTED REGIOSELECTIVE LACTONIZATION OF 2-SUBSTITUTED-1,4-BUTANEDIOLS O CH 3 O ( n- C 3 H 7 ) 4 RuO 4 Ar N Ar Ar OH O + O + CH 2 Cl 2 OH O O Ar = phenyl or 3,4-methylenedioxyphenyl 67:33 67 - 82% yield Kamlage, S.; Sefkow, M.; Zimmermann, N.; Peter, G. M. Synlett 2002 , 77. O O O RuH 2 (PPh 3 ) 4 Ar Ar Ar OH + 2 O + O + 2 OH toluene 20 ° C , 10 h Ph Ph O S/C = 25 B A A:B yield (%) Ar 3,4-dimethoxyphenyl 79 95:5 3,4-methylenedioxyphenyl 75 96:4 Shao, L.; Miyata, S.; Muramatsu, H.; Kawano, H.; Ishii, Y.; Saburi, M.; Uchida, Y. J. Chem. Soc., Perkin Trans. 1 , 1990, 1441.
6 REPORTED REGIOSELECTIVE LACTONIZATION OF POLYOLS 1 OH O RuCl 2 (PPh 3 ) 3 OH 2 6 + 2 + 2 HO acetone HO 180 ゜ OH O O 2 C, 3 h 6 1 S/C = 20 71% Murahashi, S.; Naota, T.; Ito, K.; Maeda, Y.; Taki, H. J. Org. Chem . 1987 , 52 , 4319. O O O HO OH OH RhH(PPh 3 ) 4 O OH + 2 + 2 HO HO DMF OH 80 ゜ Ph Ph C OH OH HO S/C = 2.5 90% Isaac, I.; Aizel, G.; Stasik, I.; Wadouachi, A.; Beaupere, D. Synlett 1998 , 475.
7 SYNTHESIS OF 2-ARYLMETHYL-1,4-DIOLS O O [RhCl(cod)] 2 OCH 3 OCH 3 + B(OH) 2 OCH 3 N(C 2 H 5 ) 3 , 1.0 equiv OCH 3 R n 1,4-dioxane, H 2 O (6/1) R n O rt 1.1 equiv O S/C = 33 Ar yield, % (2 steps) 92 LiAlH 4 , 3.0 equiv OH O 73 THF, reflux OH R n O CH 3 O 91 70 PhCH 2 O Itooka, R.; Iguchi,Y.; Miyaura, N. J. Org. Chem . 2003, 68 , 6000.
8 LACTONIZATION OF 2-BENZYL-1,4-BUTANEDIOL CATALYST TUNING: COUNTER ANION AND Cp STRUCTURES O Ru cat Ph Ph KO t -C 4 H 9 Ph OH + O O OH acetone 30 °C, 1 h O S/C = 100, Ru cat:KO t -C 4 H 9 = 1:1 A B [diol] = 0.5 M >99% yield OTf Ph 2 Ph 2 P P Ru cat: Ru Ru N N Cl NCCH 3 H 2 H 2 A:B* 77:23 77:23 Cl PF 6 Ph 2 Ph 2 P P Ru Ru N N NCCH 3 NCCH 3 H 2 H 2 56:44 79:21 *determined by 1 H NMR analysis
9 LIGAND EFFECT: N- ,P- AND N- α -SUBSTITUENTS OTf Ar Ar P Ar 2 P N Ar 2 P N A:B A:B Ru Ph N NCCH 3 78:22 81:19 Ru cat NH 2 Ph 2 P NH 2 Ph 2 P CH 2 Ph Ph Ar: 82:18 76:24 NHCH 3 Ph 2 P NH 2 Ph 2 P CH 2 Ph 4-tol 83:17 78:22 NHCH 2 Ph NH 2 Ph 2 P (4-tol) 2 P CH 2 Ph 2,6-xy 81:19 (2,6-xy) 2 NH 2 P
10 LIGAND EFFECT: BACKBONE O Ru cat Ph Ph KO t -C 4 H 9 Ph OH + O O OH acetone 30 °C, 1 h O S/C = 100, Ru cat:KO t -C 4 H 9 = 1:1 A B [diol] = 0.5 M A:B yield, % P N >99 82:18 Ph 2 P NH 2 OTf P >99 92:8 Ru 85 (0 °C) 93:7 N Ph 2 P NH 2 NCCH 3 Ru cat 47 92:8 Ph 2 P NCH 3 H 20 92:8 Ph 2 P NCH 2 Ph H
11 EFFECT OF ARYLMETHYL SUBSTITUENTS IN 1,4-DIOL OTf Ph 2 P Ru N NCCH 3 H 2 Ru cat S/C = 100, Ru cat:KO t -C 4 H 9 = 1:1 [diol] = 0.5 M yield, % A:B yield, % A:B OH OH >99 92:8 >99 92:8 OH OH O O OH OH >99 92:8 >99 92:8 OH OH CH 3 O PhCH 2 O
12 REPORTED SYNTHESIS OF BIOACTIVE LIGNANS O O O 1) LDA, THF/HMPA O Br O O 2) O O O O Hinokinin O O O O O 1) LHMDS, THF O CH 3 O 2) CH 3 O CHO CH 3 O OCH 3 OCH 3 CH 3 O Isodeoxypodophyllotoxin 3) TFA Bode, J. W.; Doyle, M. P.; Protopopova, M. N.; Zhou, Q. -L. J. Org. Chem. 1996 , 61, 9146.
13 SYNTHESIS OF JUSTICIDIN E O O O O Ru cat OH O O KO t -C 4 H 9 OH O O O + acetone O 30 °C, 15 h O O O O O O Justicidin E Taiwanin C S/C = 50, Ru cat:KO t -C 4 H 9 = 1:1 94% 0% OTf Ph 2 P Ru N NCCH 3 H 2 Ru cat
14 LACTONIZATION OF UNSYMMETRICAL 4-SUBSTITUTED-1,4-DIOLS primary vs tertiary O Ru cat 1 1 O OH KO t -C 4 H 9 OH + 2 O 2 + OH acetone 4 4 30 °C, 1 h CH 3 CH 3 CH 3 CH 3 diol:Ru cat:KO t -C 4 H 9 = 100:1:1 >99% [diol] = 0.5 M primary vs secondary O Ru cat 1 1 O OH KO t -C 4 H 9 OH + 2 O 2 + OH acetone 4 4 30 °C, 1 h R 1 R 2 R 1 R 2 diol:Ru cat:KO t -C 4 H 9 = 100:1:1 [diol] = 0.5 M R 1 R 2 yield, % Ph 2 P CH 3 H >99 Ru CH 3 D (96%) >99 (96% atom D) N Cl H 2 The 79th Annual Meeting of Chemical Society of Japan . 2001 , 1H326 Ru cat
15 LACTONIZATION OF TRIOLS Lactonization of 1,4,6-triol O 1 O 1 Ru cat 1 OH 1 O O KO t -C 4 H 9 OH O O 6 6 4 acetone 4 OH 4 OH 4 30 °C, 31 h HO HO 6 6 >99% 0% 0% diol:Ru cat:KO t -C 4 H 9 = 33:1:1 [diol] = 0.1 M Lactonization of 1,4,7-triol O 1 O O 1 1 OH Ru cat 7 KO t -C 4 H 9 O OH 4 7 acetone 4 7 4 OH 30 °C, 48 h OH HO >99% 0% diol:Ru cat:KO t -C 4 H 9 = 33:1:1 [diol] = 0.1 M Ph 2 P Ru N Cl H 2 Ru cat
16 LACTONIZATION OF 1,4,5-TRIOLS: SYNTHESIS OF L-FACTOR AND MURICATACIN O 1 1 Ru cat OH Ph 2 KO t -C 4 H 9 O OH 4 P acetone Ru 4 30 ゜ C, 1 h OH N Cl R OH 5 5 R H 2 triol:Ru cat = 100:1 R = C 5 H 11 [triol] = 0.5 M Ru cat >99% R = C 12 H 25 [triol] = 0.05 M O Autoregulatory factor of the antibiotic-producing O Gram-positive actinomycete Streptomyces griseus L-Factor HO Obtained from Annona muricate O O muricatacin HO Potent cytotoxic agent on tumor cell lines
17 SUMMARY Lactonization of 2-substituted-1,4-diol O Ru cat Ar Ar KO t -C 4 H 9 Ar OH + O O OH acetone O 92:8 >99% OTf Ph 2 CH 3 O P Ar = Ru N NCCH 3 H 2 O Ru cat O PhCH 2 O Regiocontrol by Cp ligand structure and PN chelate ring size 4-Substituted-1,4,n-triols OH OH n Ph 2 Ru cat n 1 P 1 KO t -C 4 H 9 OH Ru O OH N acetone Cl H 2 4 4 O (n = 1, 2, 3) Ru cat
Recommend
More recommend