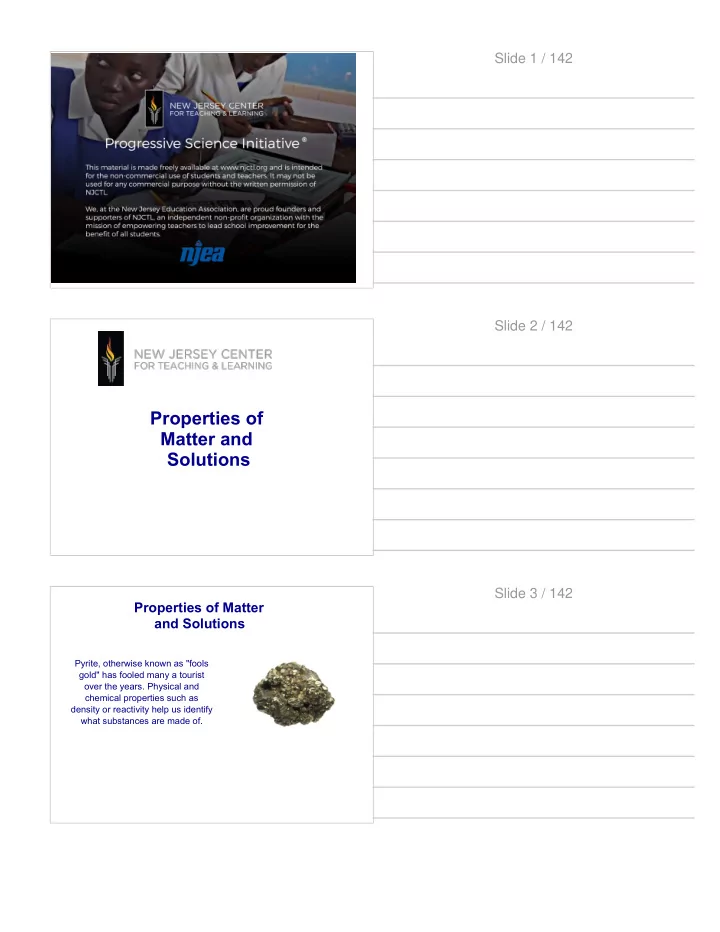

Slide 1 / 142 Slide 2 / 142 Properties of Matter and Solutions Slide 3 / 142 Properties of Matter and Solutions Pyrite, otherwise known as "fools gold" has fooled many a tourist over the years. Physical and chemical properties such as density or reactivity help us identify what substances are made of.
Slide 4 / 142 Matter We define matter as anything that has mass and takes up space. Molecules of a Atoms of an element compound Mixture of elements molecules of a and a compound diatomic element Slide 5 / 142 What is Matter Made of? Elements and Compounds Substances that could not be broken down by any physical or chemical method were/are called elements Substances that could be broken down into different elements using physical or chemical methods were/are called compounds Element Compound Ne(g) CO 2 (g) Ca(s) CaCO 3 (s) Au(s) AuNO 3 (s) Hg(l) HgI(s) Slide 6 / 142 Elements Elements are found on the periodic table. Mg-Magnesium Cu-Copper Al Na I-Iodine vapor Aluminum foil Sodium C-carbon diamond and graphite
Slide 7 / 142 Compounds Compounds are formed by combinations of different types of elements. CAFFEINE Slide 8 / 142 1 Which of the following would NOT be a compound? A HCl B CS 2 C H 2 O D CH 4 E I 2 Slide 8 (Answer) / 142 1 Which of the following would NOT be a compound? A HCl Answer B CS 2 E C H 2 O D CH 4 E I 2 [This object is a pull tab]
Slide 9 / 142 2 Which of the following is FALSE regarding compounds? A They consist of more than one element combined B A compound has a set of properties distinct from the individual elements from which it is made When a compound is separated into its elements, the elements will have the same properties of the C compound D Br 2 would not be considered a compound E NaCl would be considered a compound Slide 9 (Answer) / 142 2 Which of the following is FALSE regarding compounds? A They consist of more than one element combined B A compound has a set of properties distinct from Answer the individual elements from which it is made C When a compound is separated into its elements, the elements will have the same properties of the C compound D Br 2 would not be considered a compound [This object is a pull tab] E NaCl would be considered a compound Slide 10 / 142 Law of Definite Composition When electricity is passed through water (a compound), hydrogen and oxygen gas are produced. electricity liquid water ------------> hydrogen gas + oxygen gas 100 grams 11.2 grams 88.8 grams When the amounts of gases produced are analyzed, no matter where the water came from or how large the sample, water always consists of exactly 11.2% hydrogen and 88.8% oxygen by mass.
Slide 11 / 142 Law of Definite Composition In fact, each compound had it's own definite composition by mass. % carbon by % oxygen by Substance mass mass carbon dioxide 27.3% 72.7% carbon monoxide 42.8% 57.1% This principle, that a certain substance will have it's own unique set composition of elements, is known as the Law of Definite Composition. Slide 12 / 142 Pure Substances vs. Mixtures Some matter can be separated by heat, filtering, or boiling into other substances but did NOT obey the law of definite composition. These substances are known as mixtures and are NOT pure substances. More on mixtures later! Pure Substance Mixture Non-definitive composition Definitive Composition Examples: Examples: gold (Au) steel (Fe, C, Mn, Cr, ...) salt water (H 2 O, Cl-, Na + , ...) pure water (H 2 O) Slide 13 / 142 3 A sample of material A is collected in Nevada and found to consist of 94% oxygen and 6% hydrogen by mass. Another sample of material A is collected in Maine and found to contain 94% oxygen and 6% hydrogen. What kind of substance is this? A Element B Compound C Mixture D B and C E A, B, and C
Slide 13 (Answer) / 142 3 A sample of material A is collected in Nevada and found to consist of 94% oxygen and 6% hydrogen by mass. Another sample of material A is collected in Maine and found to contain 94% oxygen and 6% hydrogen. What kind of substance is this? Answer A Element B B Compound C Mixture D B and C [This object is a pull tab] E A, B, and C Slide 14 / 142 4 A sample of a material is found to contain 56% oxygen, 32% iron, and 12% sulfur. When another sample of the same material is collected, the composition was 44% oxygen, 30% iron, and 25% sulfur. What kind of substance is this? A element B compound C mixture D pure substance E B and D Slide 14 (Answer) / 142 4 A sample of a material is found to contain 56% oxygen, 32% iron, and 12% sulfur. When another sample of the same material is collected, the composition was 44% oxygen, 30% iron, and 25% sulfur. What kind of substance is this? A element Answer C B compound C mixture D pure substance [This object is a pull E B and D tab]
Slide 15 / 142 Properties of Matter It was clear, even to the ancients, that not all matter shares the same characteristics/properties. Substance Property lustrous, soft metal, non-reactive, gold solid at room temperature transparent, liquid at room salt water temperature, could be separated by heat, no definite composition transparent, liquid at room pure water temperature, definite composition, could be separated by electrolysis solid at room temperature, high calcium melting point, non-lustrous, could carbonate be separated by heat Slide 16 / 142 Physical Properties of Matter A physical property is a characteristic that can be observed WITHOUT altering the identity of the material. Physical Properties of water water melts at 0 Celsius at standard pressure water is transparent water has a density of roughly 1 g/mL at 25 C water is not soluble (does not dissolve) in gasoline water is colorless Notice all of these properties can be observed without changing the identity of the water - it is still water! Slide 17 / 142 Physical Properties of Matter Who doesn't like brick oven pizza! A brick used in an oven is made of a mixture of aluminum oxide and silicon oxide. Think of as many physical properties of a brick that you can. Feel free to use terms like high and low if you don't know an exact number. high density high melting point move for answer reddish color brittle (break not bend)
Slide 18 / 142 5 Which of the following IS NOT a physical property? A copper has a reddish gold color B iron reacts with oxygen to form rust C table salt dissolves easily in water D silver is an excellent conductor of electricity E all of theses are physical properties Slide 18 (Answer) / 142 5 Which of the following IS NOT a physical property? A copper has a reddish gold color B iron reacts with oxygen to form rust Answer B C table salt dissolves easily in water D silver is an excellent conductor of electricity E all of theses are physical properties [This object is a pull tab] Slide 19 / 142 6 Which of the following IS a physical property? A acetone has a density of 0.87 g/mL B aluminum will burn in air to make aluminum oxide C water can undergo electrolysis and produce hydrogen and oxygen gas D Both A and C E Both B and C
Slide 19 (Answer) / 142 6 Which of the following IS a physical property? A acetone has a density of 0.87 g/mL B aluminum will burn in air to make aluminum oxide Answer C water can undergo electrolysis and produce A hydrogen and oxygen gas D Both A and C E Both B and C [This object is a pull tab] Slide 20 / 142 Physical and Chemical Changes Physical Changes Chemical Changes Chemical changes Changes in matter result in new that don't change the composition of a substances. substance. Includes combustion, Includes changes of oxidation, state, temperature, decomposition, etc. volume, etc. Slide 21 / 142 Chemical Properties These properties can only be observed when we attempt to change the identity of the material. There are a few tell tale signs that a chemical change has taken place: Color change Emission of Light Precipitate formation Production of gas
Slide 22 / 142 Chemical Properties Color change - marshmallow burning Emission of Light - wood burning Slide 23 / 142 Chemical Properties Precipitate formation - solid forming from liquid mixtures + Production of gas - when limestone is heated heat + Slide 24 / 142 Chemical Properties Class Discussion Compare the chemical properties of a pepperoni pizza with that of the brick oven. The pizza will react with the oxygen in the air and burn. The move for answer brick will not burn in the air.
Slide 25 / 142 7 Which of the following is NOT a chemical property? A Silver tarnishing into silver oxide B gasoline burning in air C candle wax burning D candle wax melting E iron rusting Slide 25 (Answer) / 142 7 Which of the following is NOT a chemical property? A Silver tarnishing into silver oxide Answer B gasoline burning in air D C candle wax burning D candle wax melting E iron rusting [This object is a pull tab] Slide 26 / 142 8 All of the following are physical properties except….? A Gold's low reactivity with oxygen B Gasoline's inability to dissolve in water C Water melting at 0 C D Hot knife cutting through ice cream cake E evaporating water away from salt water
Recommend
More recommend