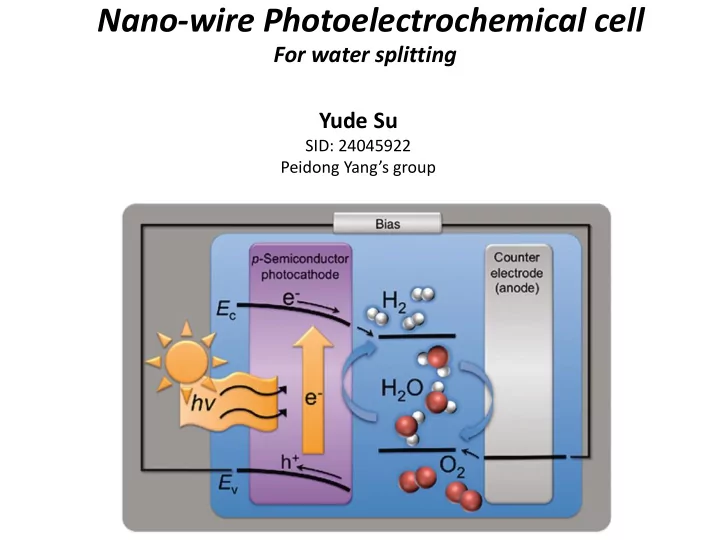

Nano-wire Photoelectrochemical cell For water splitting Yude Su SID: 24045922 Peidong Yang’s group
Outline 1. Motivation of PEC 2. Mechanism of PEC 3. Some examples 4. Future direction
Motivation • Renewable Energy replace Fossil Fuel 264 GW 70 GWe (2008) (2011) Car running on Solar (2012) Car running on Biomass (WW II)
Motivation η 1 >20% Photovoltaic cell Electricity η 2 >80% H 2 and O 2 η 3 <<1% H 2 O Photocatalyst Photochemical cell
Overall picture of a PEC cell PEC PV Device + Electrochemical Reaction Chem. Rev. 1992, 92, 411-433
Schottky contact: M/pS with M < S before contact after contact qV i q q M q S q q S q M E CB E CB E FS E FM E FM E VB q B E FS qV i E VB 2 V i W W qN A + - - - - + + + 6
Ohmic contact: M/nS with M > S before contact after contact q q S q M q M q S q E CB E FM E FM E FS E CB E FS E VB E VB + - - + - - + + 7
light emission from semiconductors - - - - - - - CB - - - - - - - - - - - - + + + + + + excitation steady emission + + + + + + + + + + + VB + + excitation off - - - - - after time - - - - - + + + transient emission + + + + + + + 8
Electrode Kinetics Butler-Volmer Equation Current-Overpotential link nF / RT (1 ) nF / RT J J e [ e ] 0 1 J k FC ( x 0) C ( x 0) 0 0 η: over -potential, defined by E-E eq J 0 : exchange current density K 0 : the standard rate constant, defined by the activation energy when k O =k R α: the transfer coefficient
Equivalent Circuit for a PEC cathode V Cathode Counter E2 E2 Electrode O Mass transport R E Eq R O E C E1 Butler-Volmer Term Behave as a Series Resistance in the PEC Equivalent Circuit ! q V ( )/ KT q V ( )/2 KT I I e ( 1) I ( e 1) ( V ) / R I s RG sh L nF RT nF RT / ( 1) / I e [ e ] 0
How to define the efficiency of a PEC cell? 5 Roughness factor=100 Red: Methyl Viologen 0 Purple: H2 evolution 100%Pt coating Blue: H2 evolution 50%Pt coating Green: H2 evolution 10%Pt coating Cyan-blue: H2 evolution 1%Pt coating -5 Current(mA) Yellow: H2 evolution 0.1%Pt coating Brown: H2 evolution 0.01%Pt coating -10 -15 -20 -0.2 -0.1 0 0.1 0.2 0.3 0.4 0.5 Voltage(V vs Solution) V OC : Open circuit voltage I SC : Short circuit current P / V * I FF: Fill factor= Max OC SC P / P Max input
Why to choose Nanowire as the PEC electrode? 1. Larger surface area, more catalytic sites 2. Strong scattering increases light path (Surface chemistry) (Optical absorption) Y. J. Hwang, et. al., Nano Lett. , 2009 , 9 , 410 M. D. Kelzenberg, et. al., Nature Mater ., 2010 , 9 , 239 3. Orthogonalize absorption and charge collection Resultant improvement of photoelectrode (Charge transport) J. M. Foley, et. al., Energy Environ. Sci. , 2012 , 5 , 5203 K. Sivula, et. al., ChenSusChem , 2011 , 4 , 432
Nanowire for PEC cathode Si GaP S. W. Boettcher, et. al., Science , 2010 , 327 , 185 J. Sun, et. al., J. Am. Chem. Soc ., 2011 , 133 , 19306 M. D. Kelzenberg, et. al., Nature Mater ., 2010 , 9 , 239 Nanowire for PEC anode ZnO TiO2 B. Liu, et. al., J. Am. Chem. Soc. , 2009 , 131 , 3985 X. Yang, et. al., Nano Lett ., 2009 , 9 , 2331 I. S. Cho, et. al., Nano Lett ., 2011 , 11 , 4978 H. M. Chen, et. al., Angew. Chem. Int. Ed , 2010 , 49 , 5966 M. Xu, et. al., Nano Lett. , 2012 , 12 , 1503
Cathode and anode integration Z-scheme Energy Diagram Current matching
Future direction 1. Develop earth-abundant semiconductor material 2. Improve the efficiency of photo-anode 3. Further increase the light absorption 4. Decrease the synthesis price Energy crisis can be solved! Thanks for your attention
Recommend
More recommend