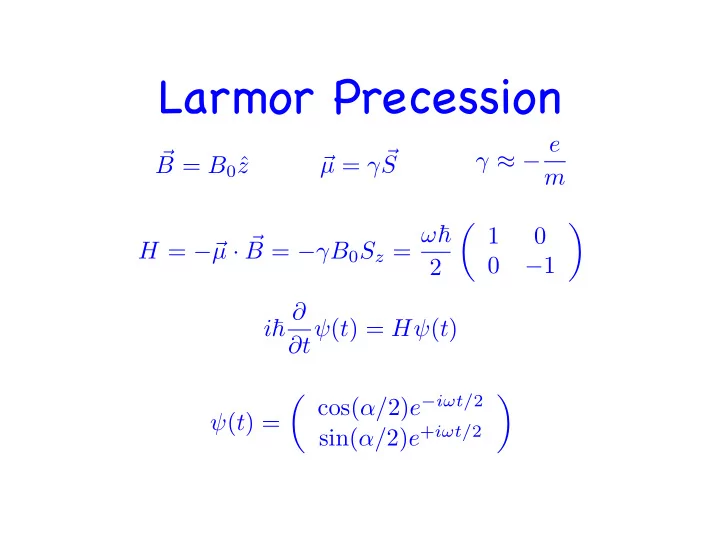

Larmor Precession γ ≈ − e µ = γ� � � S B = B 0 ˆ z m � 1 � B = − γ B 0 S z = ω � 0 µ · � H = − � 0 − 1 2 i � ∂ ∂ t ψ ( t ) = H ψ ( t ) � cos( α / 2) e − i ω t/ 2 � ψ ( t ) = sin( α / 2) e + i ω t/ 2
Larmor Precession � 1 cos( α / 2) e i ω t/ 2 sin( α / 2) e − i ω t/ 2 � � � 0 � � ψ | S z | ψ � = 0 − 1 2 � cos( α / 2) e − i ω t/ 2 � × sin( α / 2) e i ω t/ 2 � cos 2 ( α / 2) − sin 2 ( α / 2) � � = 2 � = 2 cos( α )
Larmor Precession � 0 cos( α / 2) e i ω t/ 2 sin( α / 2) e − i ω t/ 2 � � � 1 � � ψ | S x | ψ � = 1 0 2 � cos( α / 2) e − i ω t/ 2 � × sin( α / 2) e i ω t/ 2 � e i ω t + e − i ω t � � = 2 cos( α / 2) sin( α / 2) � = 2 sin( α ) cos( ω t )
Larmor Precession � 0 cos( α / 2) e i ω t/ 2 sin( α / 2) e − i ω t/ 2 � � � − i � � ψ | S y | ψ � = i 0 2 � cos( α / 2) e − i ω t/ 2 � × sin( α / 2) e i ω t/ 2 � − e i ω t + e − i ω t � � = 2 cos( α / 2) sin( α / 2) i � = 2 sin( α ) sin( − ω t )
Larmor Precession � � ψ | S x | ψ � = 2 sin( α ) cos( ω t ) � � ψ | S y | ψ � = 2 sin( α ) sin( − ω t ) � � ψ | S z | ψ � = 2 cos( α ) ω = − γ B 0
Stern-Gerlach Nobel Prize 1943
Stern-Gerlach H = − γ � B · � S � ∇ · � � B = − α x ˆ x + ( B 0 + α z )ˆ z B = 0 silver atom rest frame, x=0 0 t < 0 H = − γ ( B 0 + α z ) S z 0 ≤ t ≤ T 0 T < t χ ( t < 0) = a χ ↑ + b χ ↓ χ (0 < t < T ) = a χ ↑ e − i ω t/ 2+ i γα zt/ 2 + b χ ↓ e i ω t/ 2 − i γα zt/ 2 ω = − γ B 0
Stern-Gerlach χ ( t > T ) = a χ ↑ e − i ω T/ 2+ i γα zT/ 2 + b χ ↓ e i ω T/ 2 − i γα zT/ 2 p z = ± αγ T � 2
Hydrogen
Helium
Lithium
Elements Z protons ignoring repulsion, ( n, l, m ) orbitals 2 electrons per orbital ↑↓ (spin singlet) n 2 -fold degeneracy n = 1 2 electrons 2 2 · 2 = 8 electrons n = 2 3 2 · 2 = 18 electrons n = 3 4 2 · 2 = 32 electrons n = 4 5 2 · 2 = 50 electrons n = 5 . . . . . .
2 2 8 8 8 18 18 32 18 50
Elements Z Element outer electrons 1 Hydrogen 1 in (1,0,0) 2 Helium 2 in (1,0,0) 3 Lithium 1 in (2,0,0) 4 Beryllium 2 in (2,0,0) } 5 Boron 1 in (2,1,m) 6 Carbon 2 in (2,1,m) (2 l +1)x2=6 7 Nitrogen 3 in (2,1,m) 8 Oxygen 4 in (2,1,m) 9 Fluorine 5 in (2,1,m) 10 Neon 6 in (2,1,m) 11 Sodium 1 in (3,0,0) 12 Magnesium 2 in (3,0,0) } 13 Aluminum 1 in (3,1,m) 14 Silicon 2 in (3,1,m) (2 l +1)x2=6 15 Phosphorous 3 in (3,1,m) 16 Sulfur 4 in (3,1,m) 17 Chlorine 5 in (3,1,m) 18 Argon 6 in (3,1,m) 19 Potassium 1 in (4,0,0) 20 Calcium 2 in (4,0,0) 21 Scandium 1 in (3,2,m) . . . . . . . . .
Elements 2s+1 L J 36 Kr Krypton [Ar](4s) 2 (3d) 10 (4p) 6 1 S 0 47 Ag Silver [Kr](4d) 10 (5s) 1 2 S 1/2
Recommend
More recommend