
Ex Extract ractio ion The British Pharmacopoeia contain a number of extraction of vegetable drugs. Also, the starting material for some products such as steroids may come from natural sources Extraction can be defined as: the removal of soluble material from an insoluble residue, either liquid or solid, by treatment with a liquid solvent. It is, therefore, a solution process. The controlling factor in the rate of extraction is normally the rate of diffusion of the solute through the liquid boundary layer or layers at the interface.
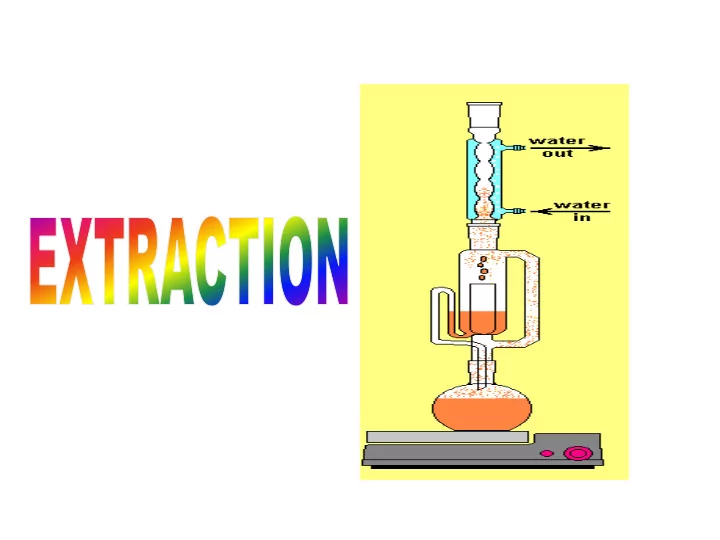
LIQ IQUID ID/LIQ IQUID ID EX EXTRACTIO ION Principle of liquid/liquid extraction 1. A solution of the substance to be extracted is brought into contact with another solvent for the substance that is immiscible with the first solvent. 2. A concentration gradient is set up between the phases, and mass transfer will occur until an equilibrium is established. 3. The distribution of the solute depending on the distribution coefficient .
4. As the process is controlled by mass transfer, the liquids must be thoroughly mixed to give a large enough surface area for contact, sufficient time for equilibrium to be set up and, finally, the liquids are separated.
Example of small-scale use of liquid/liquid extraction is the purification procedures used in alkaloidal assays . This can be achieved using separating funnel in the laboratory (Small Scale) and using stirred tanks in large- scale . Columns may be used for extraction process. In pharmaceutical processes centrifugal extractors, which ensure contact of the liquids in countercurrent flow, followed by rapid separation, are commonly employed.
The Po The Podbielniak elniak Ex Extr trac actor tor Principle of Operation The body of which consists of a drum mounted on a horizontal shaft and containing a number of concentric perforated cylinders. The liquids enter through the shaft, where the light liquid being taken to the periphery of the drum, while the heavy liquid enters at the axis of the shaft. Centrifugal action due to the rotation of the drum causes the heavy liquid to move towards the outside, so that the light liquid is displaced towards the shaft, both escaping again through the shaft.
Advantage of the use of centrifugal force Mass transfer coefficients are high and the perforated cylinders causing good dispersion and contact as the liquids move in countercurrent flow. The ability to deal with liquids that tend to emulsify or which have only a small difference in density. Liquid hold-up is low and processing time is short.
Disadvantages The equipment is expensive, but is justified by the high cost of many of the medicaments Application The method is used widely in the purification of many antibiotics where multistage extraction and centrifugal extractors are used for minimum of decomposition.
SO SOLID ID/LIQ IQUID ID EX EXTRACT CTIO ION Leaching is the extraction of a soluble constituent from a solid by a solvent. Although fewer preparations are now made by extracting the active constituents of vegetable drugs, the process is still of considerable importance and has more problems than liquid/liquid extraction.
Difficult culties ies of of Extract racting ng Vege getable ble Drugs • There are great variations in the characters of vegetable drugs, some are soft and spongy and can be extracted easily in the whole state, while others are very hard and tough (certain seeds). • There are considerable differences in the active constituents to be extracted; alkaloids, glycosides, tannins, resins or oils. • Different forms of insoluble matter may affect the extraction process, as the cellulose of the cell structure, and proteins or carbohydrates such as starch.
• In many drugs the active constituent is not the only soluble material present and a drug may contain a small proportion of an alkaloid with a large proportion of sugars. In this case, a solvent is chosen that is selective to dissolve the alkaloid only. • Wet vegetable material is an excellent medium for microbial growth and, if allowed to occur, this may lead to loss of constituents and deterioration of the product. Thus the solvent must have suitable preservative properties.
All extraction processes have common stages Suitable size reduction of the drug. Penetration of the drug by the solvent. Solution of the soluble matter within the cells. The escape of the dissolved material through the cell walls and through the solvent boundary layer surrounding the particles of the drug. Finally, separation of the solution and the exhausted drug.
SIZE REDUCTION OF THE DRUG The appropriate degree of size reduction will cause large enough surface area for adequate mass transfer: Cause some of the cells of the drug to be broken to assist penetration of the solvent and escape of soluble matter. Provide a particle size that will not result in a very long path for the solvent and the soluble matter. This distance will be equivalent to the radius of a sphere, or the shortest distance from any point in the interior to the surface in particles of irregular shape.
For excellent mass transfer, the maximum surface area should be obtained till the individual cells, but in practice this is not possible because: It would be very difficult to size reduce materials to this extent. A prolonged size reduction process may lead to decomposition of constituents or loss of volatile materials. A suspension of extremely fine particles would form an unfilterable slime which is very difficult to be separated in the final stages.
Breakage of all cells releases the entire cell contents, including inert materials, so that dry extracts are diluted. Therefore it is preferable to keep many of the cell walls unbroken to act as a filter for the retention of insoluble matter or colloidal materials.
The degree of size reduction to be used will depend on the botanical structure of the drug: Ranging from a sliced for soft drugs (such as gentian) Coarse for materials such as cascara or belladonna Moderately fine Powder for a hard and woody drug as ipecacuanha. It has been shown that, if dry extracts are made from drugs such as belladonna or stramonium, it is possible to obtain a greater total extractive when finer grades of powder are used, but a higher proportion of alkaloid when a moderately Coarse Powder are used.
PENETRATION OF THE SOLVENT INTO THE DRUG A drug in the dry state is porous due to shrinkage, and the pores contain air that must be displaced as the solvent enters into the pores and penetrates into the cells. When the dry drug is moistened, the tissues swell, the amount of swelling is variable, being greatest with liquids when hydroxyl groups form a great part of the molecule. Thus, water causes considerable swelling, while is much less with ethanol. Glycerol will lead to swelling as that obtained with water but the greater viscosity of glycerol causes the liquid to be taken up much more slowly.
The swelling continues until the pressure caused by the liquid layers cause distension and bursting of thin-walled cells that have taken up liquid by osmosis. As the solvent must displace air from pores, this can be aided by using a vacuum pump. When the pressure is reduced the atmosphere forces solvent into the drug, which facilitate penetration.
SOLUTION OF CONSTITUENTS Once the solvent has penetrated into the cells, solution of the constituents takes place and the rate of solution is increased by elevation of temperature.
ESCAPE OF THE SOLUTION FROM THE CELLS During the size reduction process, some cells are broken open, while others are damaged or distorted and, where this occurs, the escape of the solution is not hindered. However many cells remain whole, so that the soluble matter must pass through the walls. Thus, the rate of diffusion will depend on: The presence of a suitable concentration gradient through the boundary layer The thickness of the boundary layer The diffusion coefficients of the solutes in the solvent.
Factors affecting the rate of extraction and controlling mass transfer: 1. Where the drug is immersed in a quantity of solvent: (a)By agitating the mixture occasionally, which disperses local concentrations of the solution, thus increasing the concentration gradient. (b)By agitating the solvent and the drug continuously, which reduces the thickness of the boundary layer. (c)By suspending the drug in a cloth bag near to the surface of the liquid. As the constituents dissolve, the density of the solution increases, so that convection currents are established, leading to circulation of the solution.
2. If solvent flows past the particles: (a) The flow replaces the solution by pure solvent, causing an increase in the concentration gradient. (b) The spaces between the particles of drug form passages through which the solvent flows; due to the small size of these capillaries, the velocity of the solvent is sufficient to reduce the boundary layers, so increasing the concentration gradient. In both 1 and 2, the extraction can take place at elevated temperatures, if the material is thermostable and if the solvent is not affected by heating.
Recommend
More recommend