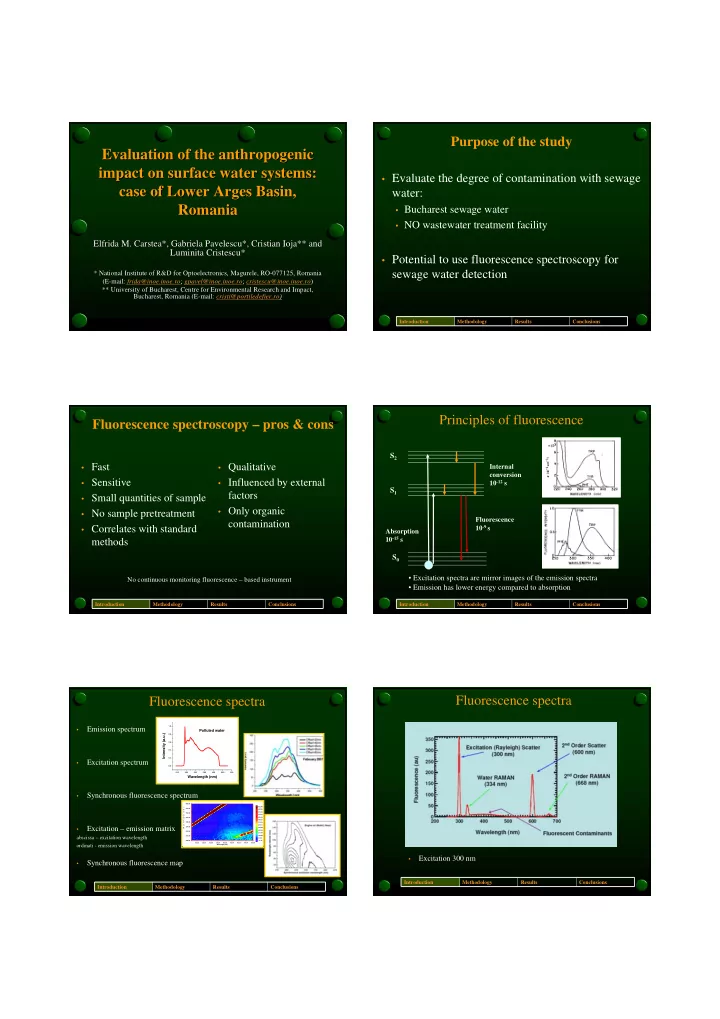

Purpose of the study Evaluation of the anthropogenic Evaluation of the anthropogenic impact on surface water systems: impact on surface water systems: • Evaluate the degree of contamination with sewage case of Lower Arges Basin, case of Lower Arges Basin, water: Romania Romania • Bucharest sewage water • NO wastewater treatment facility Elfrida M. Carstea*, Gabriela Pavelescu*, Cristian Ioja** and Elfrida M. Carstea*, Gabriela Pavelescu*, Cristian Ioja** and Luminita Cristescu* Luminita Cristescu* • Potential to use fluorescence spectroscopy for sewage water detection * National Institute of R&D for Optoelectronics, Magurele, RO- * National Institute of R&D for Optoelectronics, Magurele, RO -077125, Romania 077125, Romania (E- (E -mail: mail: frida@inoe.inoe.ro frida@inoe.inoe.ro ; ; gpavel@inoe.inoe.ro gpavel@inoe.inoe.ro ; ; cristescu@inoe.inoe.ro cristescu@inoe.inoe.ro ) ) ** University of Bucharest, Centre for Environmental Research and Impact, ** University of Bucharest, Centre for Environmental Research an d Impact, Bucharest, Romania (E- -mail: mail: cristi@portiledefier.ro cristi@portiledefier.ro) ) Bucharest, Romania (E Introduction Methodology Results Conclusions Principles of fluorescence Fluorescence spectroscopy – pros & cons S 2 • Fast • Qualitative Internal conversion • Sensitive • Influenced by external 10 -12 s S 1 factors • Small quantities of sample • Only organic • No sample pretreatment Fluorescence contamination • Correlates with standard 10 -9 s Absorption 10 -15 s methods S 0 • Excitation spectra are mirror images of the emission spectra No continuous monitoring fluorescence – based instrument • Emission has lower energy compared to absorption Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions Fluorescence spectra Fluorescence spectra 1.0 Emission spectrum • Polluted water Intensity (a.u.) 0.8 0.6 0.4 0.2 Excitation spectrum • 0.0 250 300 350 400 450 500 550 Wavelength (nm) Synchronous fluorescence spectrum • 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 1000 800 140 400.00 500 800 800 300 700 140 120 120 250 962.48 W a v e l e n g t h ( n m ) 375.00 500 100 200 120 887.43 800 800 800 800 800 800 800 800 120 800 800 800 800 800 800 800 800 800 800 800 800 200 800 800 800 800 800 600 600 100 100 400 250 812.38 350.00 600 200 600 100 600 400 737.33 Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) 500 100 80 150 150 325.00 600 600 80 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 600 80 600 600 200 662.28 150 300 80 80 400 400 587.23 300 60 400 400 300.00 150 400 100 512.18 60 60 100 400 400 400 300 400 400 400 400 400 400 400 400 400 400 400 100 400 400 400 400 400 400 400 200 400 400 400 400 60 60 437.13 Excitation – emission matrix 275.00 • 200 362.09 40 100 40 40 200 200 40 200 50 200 40 287.04 250.00 50 200 50 200 200 200 200 200 200 100 200 200 200 200 200 200 200 200 200 200 200 200 200 200 200 200 200 200 200 100 50 211.99 20 20 100 20 20 20 abscissa – excitation wavelength 225.00 136.94 0 0 0 0 0 0 0 0 0 61.89 200.00 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 300 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 350 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 400 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 450 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 500 -13.16 300.00 325.00 350.00 375.00 Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) Wavelength (nm) 400.00 425.00 450.00 475.00 500.00 ordinati - emission wavelength Wavelength (nm) Excitation 300 nm • Synchronous fluorescence map • Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions
Natural Organic Matter (NOM) Natural Organic Matter (NOM) comprises the decay products of animal and plant matter. • NOM: • Dissolved Organic Matter • Autochthonous – microbially derived • Allochthonous – terestrially derived Proteins Humic substances Natural Organic Matter Tryptophan Tyrosine Phenylalanine Humic acid Fulvic acid Dissolved Organic Matter Particulate Organic Matter Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions Methodology Methodology 1 – Sabar River • 2 – Colibasi on Arges • River 3 – Hotarele on Arges • River 4 – Budesti on Dambovita • River 5 – Soldanu on Arges • River 6 – Clatesti on Arges • NO water treatment facility River 7 – Sampling point on • Danube 8 –Dambovita River • 9 –Dambovita River • Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions Methodology Results 1.0 1.0 Polluted water Sabar Hotarele Intensity (a.u.) 0.8 • Samples taken every Colibasi 0.8 Clatesti 0.6 Intensity (a.u.) season Budesti (morning) Budesti (noon) 0.4 0.6 • Measured within 24 h February 2007 0.2 from collection 0.4 Preserved at aprox. 4 0 C 0.0 • 0.2 250 300 350 400 450 500 550 Wavelength (nm) 0.0 300 350 400 450 500 • Spectrofluorimeter PerkinElmer LS 55 Spectrofluorimeter PerkinElmer LS 55 Wavelength (nm) • Portable spectrograph Ocean Optics USB2000– – FL FL • Portable spectrograph Ocean Optics USB2000 • • Pulsed light source Xenon PX- Pulsed light source Xenon PX -2. 2. • • Q- Q -switched switched YAG:Nd YAG:Nd Laser Laser • Second, third, forth harmonics • Second, third, forth harmonics • • 10 Hz repetition rate 10 Hz repetition rate • • 4- 4 -6 ns pulse duration 6 ns pulse duration Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions
Results Results Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions Results Results Hourly evaluation Seasonal evaluation Introduction Methodology Results Conclusions Introduction Methodology Results Conclusions Conclusions Significant contamination with wastewater discharged from Bucharest, especially • at Budesti. An hourly organic matter trend connected to increased human activity in • morning and afternoon hours. • The usefulness of fluorescence spectroscopy in the quick evaluation of pollution for the water management (Amelene) Introduction Methodology Results Conclusions
Recommend
More recommend