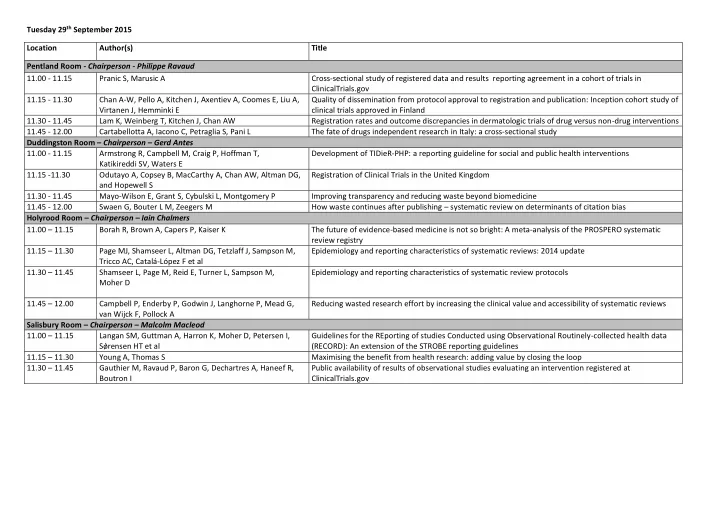

Tuesday 29 th September 2015 Location Author(s) Title Pentland Room - Chairperson - Philippe Ravaud 11.00 - 11.15 Pranic S, Marusic A Cross-sectional study of registered data and results reporting agreement in a cohort of trials in ClinicalTrials.gov 11.15 - 11.30 Chan A-W, Pello A, Kitchen J, Axentiev A, Coomes E, Liu A, Quality of dissemination from protocol approval to registration and publication: Inception cohort study of Virtanen J, Hemminki E clinical trials approved in Finland 11.30 - 11.45 Lam K, Weinberg T, Kitchen J, Chan AW Registration rates and outcome discrepancies in dermatologic trials of drug versus non-drug interventions 11.45 - 12.00 Cartabellotta A, Iacono C, Petraglia S, Pani L The fate of drugs independent research in Italy: a cross-sectional study Duddingston Room – Chairperson – Gerd Antes 11.00 - 11.15 Armstrong R, Campbell M, Craig P, Hoffman T, Development of TIDieR-PHP: a reporting guideline for social and public health interventions Katikireddi SV, Waters E 11.15 -11.30 Odutayo A, Copsey B, MacCarthy A, Chan AW, Altman DG, Registration of Clinical Trials in the United Kingdom and Hopewell S 11.30 - 11.45 Mayo-Wilson E, Grant S, Cybulski L, Montgomery P Improving transparency and reducing waste beyond biomedicine 11.45 - 12.00 Swaen G, Bouter L M, Zeegers M How waste continues after publishing – systematic review on determinants of citation bias Holyrood Room – Chairperson – Iain Chalmers 11.00 – 11.15 Borah R, Brown A, Capers P, Kaiser K The future of evidence-based medicine is not so bright: A meta-analysis of the PROSPERO systematic review registry 11.15 – 11.30 Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Epidemiology and reporting characteristics of systematic reviews: 2014 update Tricco AC, Catalá-López F et al 11.30 – 11.45 Shamseer L, Page M, Reid E, Turner L, Sampson M, Epidemiology and reporting characteristics of systematic review protocols Moher D 11.45 – 12.00 Campbell P, Enderby P, Godwin J, Langhorne P, Mead G, Reducing wasted research effort by increasing the clinical value and accessibility of systematic reviews van Wijck F, Pollock A Salisbury Room – Chairperson – Malcolm Macleod 11.00 – 11.15 Langan SM, Guttman A, Harron K, Moher D, Petersen I, Guidelines for the REporting of studies Conducted using Observational Routinely-collected health data S ǿ rensen HT et al (RECORD): An extension of the STROBE reporting guidelines 11.15 – 11.30 Young A, Thomas S Maximising the benefit from health research: adding value by closing the loop 11.30 – 11.45 Gauthier M, Ravaud P, Baron G, Dechartres A, Haneef R, Public availability of results of observational studies evaluating an intervention registered at Boutron I ClinicalTrials.gov
Tuesday 29 th September 2015 Location Author(s) Title Pentland Room – Chairperson – John Ioannidis 14.00 – 14.15 MacLeod MR on behalf of the IICARus and NPQIP investigators Strategies for publishers to improve the reporting of in vivo research 14.15 – 14.30 Leenaars M, Ritskes-Hoitinga M Lessons learned from the implementation of systematic reviews in laboratory animal science 14.30 – 14.45 Holman C, Diamantaras A, Grittner U,Kimmelman J, Where Have All the Rodents Gone? The Effects of Attrition in Experimental Research on Cancer and Stroke Piper SK, Siegerink B, Dirnagl U 14.45 – 15.00 Danborg PB, Simonsen AL, Hrǿbjartsson A, Gǿtzsche PC Risk of bias assessment in preclinical research supporting clinical practice 15.00 – 15.15 Sena ES, on behalf of the MultiPART consortium Multi centre animal studies: a strategy to improve the prospects of translational success 15.15 – 15.30 Offenhauser N, Kurreck C, Dirnagl U, for the Department of From policies to standards: Implementing and managing a structured ISO 9001 certified Quality System in Experimental Neurology QM Steering Group an academic translational neuroscience research lab Duddingston Room – Chairperson – Ana Marusic 14.00 – 14.15 Galipeau J, Cobey KD, Shamseer L, Moher D The Role of Publication Officers in Increasing Value and Decreasing Waste in Biomedical Research 14.15 – 14.30 Shamseer L, Barbour V, Bell-Syer S, Cumpston M, Deeks J, Developing Core Competencies for Scientific Editors of Biomedical Journals Garner P, MacLehose H, Straus S et al 14.30 – 14.45 Callender T Reporting Carbon to Reduce Waste and Improve Efficiency – The GREENER Initiative 14.45 – 15.00 Toews I, Wolff RF, Binder N, Toprak G, von Elm E, Meerpohl JJ Editorial policies of hematology and oncology journals: A cross-sectional study of author instructions 15.00 – 15.15 The Transparency and Openness Promotion (TOP) Committee The TOP Guideline to promote transparency, openness, and reproducibility 15.15 – 15.30 Rodríguez AC Making the case for creating synergy between EQUATOR and manuscript editing companies Holyrood Room – Chairperson – Doug Altman 14.00 – 14.15 Kapadia M, Moher D, Offringa M, on BEHALF OF PRISMA- Increasing value and reducing waste in child health systematic reviews and meta-analyses - PC and PRISMA-C STEERING GROUP Protocols (PRISMA-PC) and Reporting (PRISMA-C) 14.15 – 14.30 Clyburne-Sherin A, Thurairajah P, Chan WWY, Kapadia M, SPIRIT-C: Increasing research value / reducing research waste through evidence-based reporting Moher D, Klassen T, Offringa M standards for pediatric clinical trial protocols 14.30 – 14.45 Clyburne-Sherin A, Thurairajah P, Chan WWY, Kapadia M, CONSORT-C: Increasing research value / reducing research waste through evidence-based Moher D, Chan A, Klassen T, Offringa M reporting standards for pediatric clinical trials 14.45 – 15.00 Kapadia M, Thurairajah P, Terwee C, Beaton D, Offringa New evidence and consensus based reporting Instrument for the Selection of Pediatric Endpoints M in Clinical Trials: InSPECT 15.00 – 15.15 Kelly LE, Offringa M Increasing value and reducing waste in child health research through evidence-based guidance for selecting a comparator treatment in pediatric clinical trials 15.15 – 15.30 Kapadia M, Kelly LE, Needham A, Chan W, Clyburne- Increasing value and reducing waste in child health research by developing and implementing Sherin A, Thurairajah P, Offringa M novel methodology: Enhancing Research Impact in Child Health (EnRICH) Salisbury Room – Chairperson – Gerd Antes 14.00 – 14.15 Barnett A, Clarke P, Herbert D Randomness and high cost for funding outcomes 14.15 – 14.30 Shamseer L, Moher D, Maduekwe O, Turner L, Barbour V, Comparison of characteristics potential predatory journals with subscription and open access journals. A Burch R et al cross-sectional study. 14.30 – 14.45 Olivieri J, Manfredi L, Postacchini L, Tedesco S, Leoni P, Poor adherence to methodological recommendations supports biased research results in steroid-refractory Gabriella A et al chronic Graft-versus-Host Disease: a systematic review and meta-analysis 14.45 – 15.00 Malicki M, Utrobicic A, Marusic A Addressing duplicate publications in biomedicine: a mixed method study 15.00 – 15.15 Hemminki E Improper research regulation is a cause for waste in clinical research 15.15 – 15.30 Bouter LM, Axelsen N, ter Riet G, Tijdink J Survey among experts to rank major and minor research misbehaviors
Recommend
More recommend