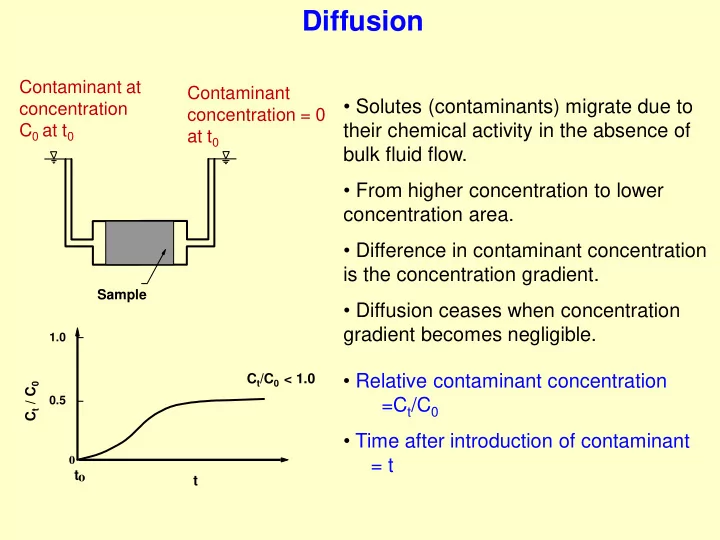

Diffusion Contaminant at Contaminant • Solutes (contaminants) migrate due to concentration concentration = 0 C 0 at t 0 their chemical activity in the absence of at t 0 bulk fluid flow. • From higher concentration to lower concentration area. • Difference in contaminant concentration is the concentration gradient. Sample • Diffusion ceases when concentration gradient becomes negligible. 1.0 • Relative contaminant concentration C t /C 0 < 1.0 C t / C 0 0.5 =C t /C 0 • Time after introduction of contaminant 0 = t t o t
Diffusion • Add small amount of dye in a fluid • Pulse gets spread out Add continuous dye-- a sharp front
Types of Diffusion • Steady State Diffusion J D =-D. .( C/ x) • Diffusion flux constant with time • Fick’s First law applicable D = diffusion coefficient [L 2 /T] = porosity C/ x = concentration gradient (i.e., change in concentration with distance) • Non Steady-state Diffusion • C x , t C x , t Concentration gradient non-uniform D • Follows Fick’s second law t x x
Chemical Energy Field • To study the mechanism(s) of contaminant transport – the intact and fractured rock samples (Gurumoorthy 2002) diffusion characteristics of the saturated and unsaturated soils (Rakesh 2005) • Investigations using the Cl - , I +2 , Cs +1 and Sr +2 in their active as well as inactive forms • Development of Diffusion Cell
CONTAMINANT TRANSPORT MODELING THROUGH THE ROCK MASS Diffusion cells C 0 C t C t Intact Rock mass (IRM) C o C t Fractured Rock mass (FRM)
CONTAMINANT TRANSPORT MODELING THROUGH THE ROCK MASS Diffusion characteristics Diffusion cells N 33 50 75 100 40 C 0 C t C t Fractured rock mass Intact rock mass 30 -4 ) 0 (x10 Intact Rock mass (IRM) 20 /C C t 10 C o 0 0 2000 4000 6000 8000 10000 0 2000 4000 6000 8000 10000 Time (s) 6 10 Diffusion time (s) Intact rock mass C t 5 Fractured rock mass 10 y=1.8 4 10 Fractured Rock mass t m =t p .N -2 3 (FRM) 10 y=1.97 2 10 7 min. 50 days 6 m thick FRM 1 10 1 10 100 75 min. 520 days 0.3 m thick IRM N (D i ) m =(D i ) p α α
Modeling Diffusion in soils using impedance spectroscopy (IS) 60 A C U B B 60 A 30 70 Diffusion cell Impedance value of the soil is measured by using LCR meter Diffusion of contaminant can be monitored by determining the change in the impedance of the soil
• Break-through curve 40 (a) 30 -4 ) C t /C 0 (x10 20 10 453 0 0 100 200 300 400 500 t (h) • The slope of the break-through curve diffusion coefficient, D • Archie’s law ( D = . m ) porosity of the geomaterials
Details of the diffusion studies d Θ Soil Sample S r w (kN/m 3 ) (%) (%) (%) WC 100 13.8 33 45.54 WC WC 80 14.0 27 37.8 100 WC 60 13.8 22 30.36 80 CS 100 14.7 29 42.63 60 CS CS 80 14.9 23 34.27 CS 60 14.4 18 25.92 Overall four cells were employed for each sample Z' measurement corresponding to 3 rd , 6 th , 9 th and 20 th day 1 M NaCl and 0.01 M SrCl 2 used as model contaminants Na + and Sr +2 analysis using AAS along the length of the cell
General observations 5000 th 9 day Time (Days) WC 60-N 4500 WC 60-N WC 80-N WC 100-N 3 6 9 5000 4000 C U 4500 3500 4000 3000 C U ) W Z' ( 3500 2500 2000 3000 ) W Z' ( 1500 2500 1000 2000 500 1500 0 1000 0 1 2 3 4 5 6 7 8 9 10 11 500 Length (cm) 0 0 1 2 3 4 5 6 7 8 9 10 11 1 Z ′ C t Contamination Length (cm) C t Influence of Saturation With time C t increases on U For a time t, Diffusion decreases with due to diffusion decreasing saturation Implies Z ′ decreases on U with time
(Normalized concentration) C t /C 0 vs Length 2 2 2 C x 2 exp( D m t / R L ) mx mx t 0 e d c 0 . cos sin C L m L L m 1 0 c c c (Diffusion coefficient) D e vs volumetric water content
Recommend
More recommend