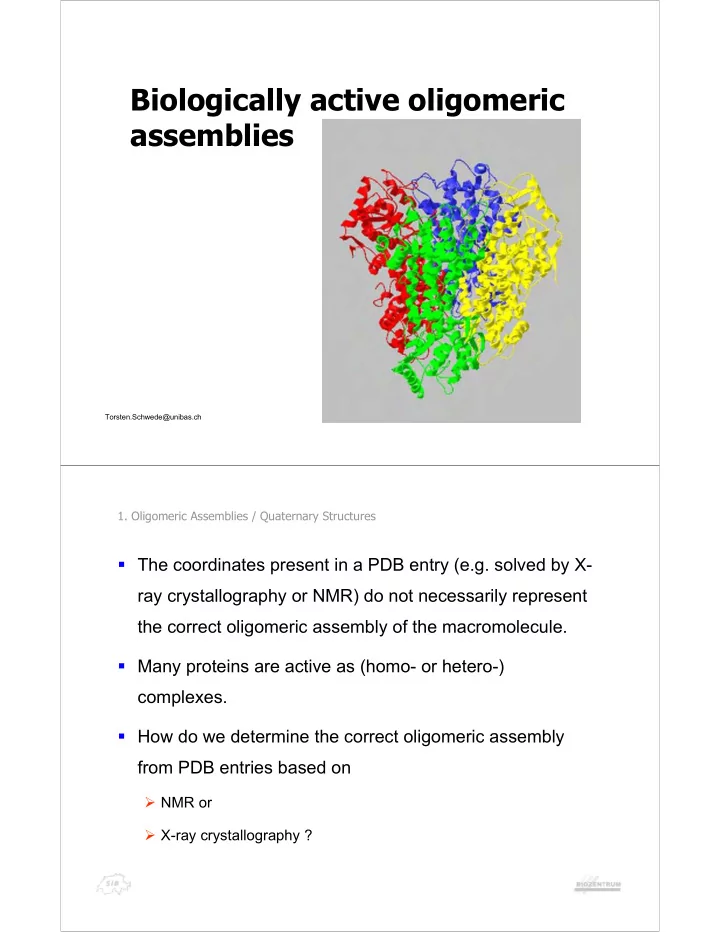

Biologically active oligomeric assemblies Torsten.Schwede@unibas.ch 1. Oligomeric Assemblies / Quaternary Structures ! The coordinates present in a PDB entry (e.g. solved by X- ray crystallography or NMR) do not necessarily represent the correct oligomeric assembly of the macromolecule. ! Many proteins are active as (homo- or hetero-) complexes. ! How do we determine the correct oligomeric assembly from PDB entries based on " NMR or " X-ray crystallography ?
1. Oligomeric Assemblies / Quaternary Structures X- -ray crystallography ray crystallography X Crystal = translated Unit Cell More than 80% of protein structures are solved by means of X-ray diffraction on crystals. An X-ray diffraction experiment produces atomic coordinates of the crystal’s Asymmetric Unit (ASU). Unit Cell = all space symmetry In general, neither ASU nor Unit Cell has any group mates of ASU relation to Biological Units, or stable protein complexes which act as units in physiological processes. Is there a way to infer Biological Unit from the protein crystallography data? PDB file (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1. Oligomeric Assemblies / Quaternary Structures Crystal interfaces Crystal interfaces Stability of protein complexes depends on properties of protein-protein interfaces, such as • free energy of formation ! G int • solvation energy gain ! G S • interface area • hydrogen bonds and salt bridges across the interface • hydrophobic specificity (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1. Oligomeric Assemblies / Quaternary Structures Interface assessment Interface assessment A crystal may be viewed as a packing of assemblies with biologically insignificant contacts between them. Protein assembly is a packing of monomeric units with biologically relevant interfaces between them. (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1. Oligomeric Assemblies / Quaternary Structures At first glance … … At first glance … the solution is simple as 1-2: 1. Evaluate all protein contacts (interfaces) in crystal 2. Leave only the strongest (“biologically relevant”) ones - and what you get will have chances to be a stable protein complex. Small technical problem: How to discriminate between “real” (biologically relevant) and “superficial” (inter-assembly, or crystal packing) interfaces? (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA Real and superficial protein interfaces Real and superficial protein interfaces Most often used 7000 dimers discrimination criteria monomers - interface area . 6000 Buried ASA [Å 2 ] 5000 A cut-off at 900 Å 2 4000 gives about 80% success rate of 3000 discrimination between monomers 2000 and dimers. 1000 Big proteins would be 0 always sticky if this criteria is true … 0 20 40 60 80 PDB entry (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Real and superficial protein interfaces Real and superficial protein interfaces Free energy gain of Free Enerfgy Gain [kcal/M] interface formation . 0 A cut-off at -8 kcal/M -20 gives about 82% success rate of -40 discrimination between monomers and dimers. -60 dimers Can energy measure monomers -80 be uniform for all weights and shapes? 0 20 40 60 80 PDB entry (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA Real and superficial protein interfaces Real and superficial protein interfaces P-value of Hydrophobic Patch dimers P-value of monomers hydrophobic patches . 0.8 A measure of probability for the 0.6 interface to be more hydrophobic than found. 0.4 A cut-off at 0.2 gives about 60% success rate of discrimination 0.2 between monomers and dimers. 0 0 20 40 60 80 PDB entry (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Real and superficial protein interfaces Real and superficial protein interfaces " No ultimate discriminating parameter for the identification of biologically relevant protein interfaces may be proposed at present even for dimeric complexes Jones, S. & Thornton, J.M. (1996) Principles of protein-protein interactions, Proc. Natl. Acad. Sci. USA , 93 , 13-20. " Formation of N>2 -meric complexes is most probably a corporate process involving a set of interfaces. Therefore significance of an interface should not be detached from the context of protein complex (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA Making assemblies from significant interfaces Making assemblies from significant interfaces Despite failure to find an ultimate measure for interface biological relevance, two approaches were developed that use scoring of individual interfaces: " PQS server @ MSD-EBI (Kim Henrick) Trends in Biochem. Sci. (1998) 23 , 358 Method: progressive build-up by addition of monomeric chains that suit the selection criteria. The results are partly curated. " PITA software @ Thornton group EBI (Hannes Ponstingl) J. Appl. Cryst. (2003) 36 , 1116 Method: recursive splitting of the largest complexes as allowed by crystal symmetry. Termination criteria is derived from the individual statistical scores of crystal contacts. The results are not curated. (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Chemical stability of protein complexes Chemical stability of protein complexes " It is not properties of individual interfaces but rather chemical stability of protein complex in general that really matters " Protein chains will most likely associate into largest complexes that are still stable " A protein complex is stable if its free energy of dissociation is positive: ! % $ ! $ ! # G diss G T S 0 int How to calculate ! G diss ? (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA Protein affinity Protein affinity Solvation Free energy Free energy Solvation energy of energies of of H-bond of salt bridge protein complex dissociated formation formation subunits n & ' & ' ( ! % ! $ ! $ $ G G A , A A G A E N E N ! int s 1 2 n s i hb hb sb sb % 1 i Number of H- Number of salt bonds between bridges between dissociated dissociated Choice of dissociation subunits: subunits subunits ! G int is function of Dissociation into protein interfaces stable subunits with minimum ! G & ' diss ) ) A A A A A A 1 2 3 1 2 3 (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Solvation free energy Solvation free energy Atom’s Atomic solvation accessible parameters surface area & ' a & ' ( r k ! % ! * $ G A a a s k k k k Atom’s accessible surface k area in reference (unfolded) state solvent Eisenberg, D. & McLachlan, A.D. (1986) Nature 319, 199-203. protein (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA Entropy of macromolecules in solutions Entropy of macromolecules in solutions Translational entropy Rotational entropy Sidechain entropy & ' & ' & ' ˆ % ) * ) S S m S I , S a trans rot S surf Solvent-accessible Mass Tensor of inertia surface area Symmetry number 3 R & ' & ' + ) S m c log m 2 trans t Murray C.W. and Verdonik M.L. (2002) & ' & ' R J. Comput.-Aided Mol. Design 16, 741-753. ˆ 2 * + ) * S I , c log I I I rot S r 2 1 2 3 S & ' + c t , c r and F are semi-empirical parameters S surf a Fa (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Entropy of dissociation Entropy of dissociation n & ' & ' ( ! % $ S S A S A , A A ! i 1 2 n % i 1 Mass of i-th subunit 2 1 . m & ' i 3 R i % $ ) ) n 1 C log / , k-th principal moment of ( 2 m 0 - i inertia of i-th subunit i 1 & ' 2 & ' . 2 2 * I A A / , k i S i R i k ) log Fa buried / , & ' & ' 2 2 2 * I A A A A ! ! 0 - k 1 n S 1 n k Fitted parameter Fitted parameter ! S is function of protein complex (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
1.1. MSD-PISA How to identify an assembly in crystal? How to identify an assembly in crystal? We now know (or we think that we know) how to evaluate chemical stability of protein complexes. Given a 3D-arrangement of protein chains, we can now say whether there are chances that this arrangement is a stable assembly, or biological unit. But how to get potential assemblies in first place? (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI) 1.1. MSD-PISA Enumerating assemblies in crystal Enumerating assemblies in crystal " crystal is represented as a periodic graph with monomeric chains as vertices and interfaces as edges " each set of assemblies is identified by engaged interface types " all assemblies may be enumerated by a backtracking scheme engaging all possible combinations of different interface types Example: crystal with 3 interface types Assembly Engaged Assembly Engaged set interface types set interface types 1 000 - only monomers 5 100 - dimer N3 2 001 - dimer N1 6 101 3 010 - dimer N2 7 110 4 011 8 111 - all crystal (slides courtesy of Eugene Krissinel & Kim Henrick, MSD-EBI)
Recommend
More recommend