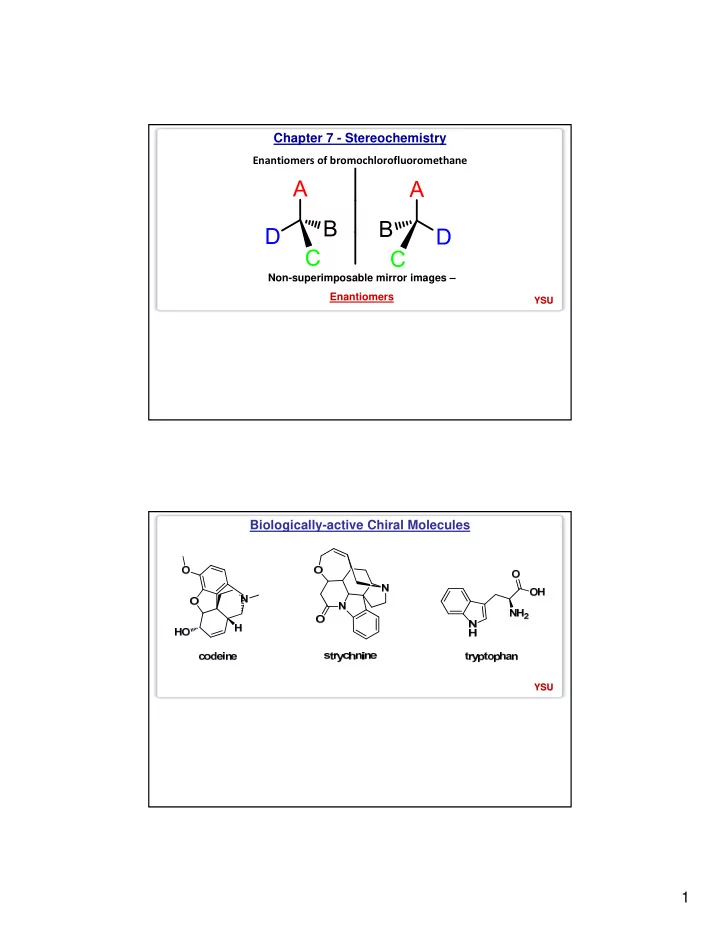

Chapter 7 - Stereochemistry Enantiomers of bromochlorofluoromethane Non-superimposable mirror images – Enantiomers YSU YSU Biologically-active Chiral Molecules YSU YSU 1
Biologically-active Chiral Molecules HO HO OH HO O O O OH HO H NH 2 HO HO OH H H L-dopa OH HO sucrose cholesterol YSU YSU $100 billion sales worldwide in 2000 Account for 32% of the $360 billion total drug sales YSU YSU 2
The Chirality Centre - Stereoisomerism Carbon atom here is asymmetric C is a stereogenic center YSU YSU 7.3 Symmetry in Achiral Structures Achiral i.e. not chiral Mirror images of chlorodifluoromethane are superimposable Figure 7.2 YSU YSU 3
7.4 Measurement of Optical Activity Typical polarimeter setup : [ ] D = 100 x (rotation)/(cell length) x (concentration) YSU YSU 7.8 Enantiomers • same physical properties except rotation of plane polarized light • one enantiomer positive rotation (+) other negative rotation ( ‐ ) YSU YSU 4
Which molecules contain chiral (stereogenic) centers? The stereogenic C must have 4 different groups attached YSU YSU 7.5 Absolute and Relative Configuration Absolute Configuration – Actual arrangement of substituents in space (+) ‐ 2 ‐ butanol and ( ‐ ) ‐ 2 ‐ butanol, but which is which? Relative Configuration ‐ Configuration relative to another compound. Pre ‐ 1951, compounds could be related to each other but the absolute configuration was not able to be determined. YSU YSU 5
7.6 Nomenclature - Use of the Cahn-Ingold-Prelog System S enantiomer R enantiomer R ‐ Rectus ‐ the clockwise arrangement of groups S ‐ Sinestre ‐ the counterclockwise arrangement of groups YSU YSU 7.6 Nomenclature - Use of the Cahn-Ingold-Prelog System YSU YSU 6
7.7 Fischer Projection Formulas Figure 7.5 YSU YSU 7.9 Reactions that create a Chirality Center Figure 7.6 YSU YSU 7
7.10 Chiral molecules with two Chirality Centers Figure 7.7 YSU YSU 7.10 Representations of (2 R , 3 R )-dihydroxybutanoic acid Conversion of “zig ‐ zag” picture to Fischer projection All the same molecule: Figure 7.8 (a) and (b) differ only by bond rotation (b) leads to correct Fischer projection YSU YSU 8
7.10 Chiral molecules with two Chirality Centers Important stereochemical labels later, particularly in carbohydrate (sugar) chemistry and biochemistry YSU YSU 7.10 Chiral molecules with two Chirality Centers Applies to other cycles, including cyclohexane; increases the molecular diversity possible using simple structures YSU YSU 9
7.11 Achiral molecules with two Chirality Centers Figure 7.9 YSU YSU Meso-2,3-butanediol Figure 7.10 YSU YSU 10
7.11 Stereogenic Centers in Cholic Acid Figure 7.11 YSU YSU 7.13 Reactions that produce diastereomers Figure 7.12 YSU YSU 11
7.13 Reactions that produce diastereomers Figure 7.12 YSU YSU 7.14 Resolution of a chiral substance into its enantiomers Figure 7.13 YSU YSU 12
Not covering 7.15 and 7.16 YSU YSU 13
Recommend
More recommend