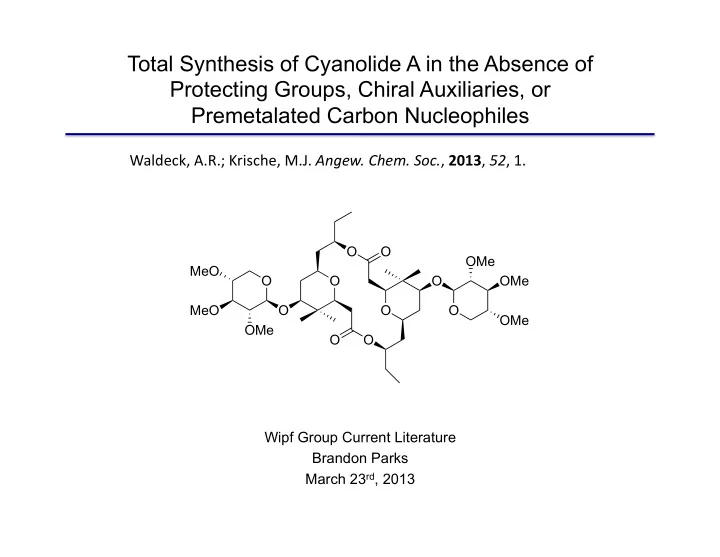

Total Synthesis of Cyanolide A in the Absence of Protecting Groups, Chiral Auxiliaries, or Premetalated Carbon Nucleophiles Waldeck, ¡A.R.; ¡Krische, ¡M.J. ¡ Angew. ¡Chem. ¡Soc. , ¡ 2013 , ¡ 52 , ¡1. ¡ O O OMe MeO O O O OMe MeO O O O OMe OMe O O Wipf Group Current Literature Brandon Parks March 23 rd , 2013
Cyanolide A Ø Isolated cyanobacterium Lyungbya bouillonii in 2010 Ø Related to the clavoslide family of natural products Ø Possesses significant molluscicidal activity (LC 50 = 1.2 µ M) against Biophalaria glabrata (water snail) involved in schistosomiasis O O OMe MeO O O O OMe MeO O O O OMe OMe O O Pereira, A.R.; McCue, C.F.; Gerwick, W.H. J. Nat. Prod. 2010 , 73 , 217.
Schistosomiasis Ø Caused by a parasitic worm often carried by Biophalaria glabrata (water snail) – ~200 million people are currently infected Ø Leads to organ damage, impaired growth and cognitive development in children, associated with increased risks of bladder cancer Ø Several treatments are known but have several side-effects O NO 2 O N Cl N H Cl N O OH praziquantel niclosamide Chitsulo, L.; Engels, D.A.; Montresor, S.L. Acta Trop . 2000 , 77 , 41.
Floreancig Retrosynthesis of Clavsolide A dimerization O O OMe MeO O OPG O O O OMe X MeO O O O OMe OMe OH O O oxidative cyclization H O OPG HO OPG LG + X X OAc Peh, G.; Floreancig, P.E. Org. Lett. 2012 , 14 , 5614.
Precursor Synthesis OTIPS Et 2 Zn, Pd(OAc) 2 H OMs Ph 3 P HO O OTIPS + CH 2 Cl 2 , -20 °C H 82% TMS TMS 8:1 d.r., 85% ee A OMs OTIPS OTIPS H H 1. NaH, 15-C-5, THF; O AcO O A , 0 °C, 63% + 2. [( p -cymene)RuCl 2 ] 2 HOAc, Na 2 CO 3 , toluene OAc OAc 80 °C, 6 ( B , 55%) or 12 h ( C , 69%) C B [CpRu(NCCH 3 ) 3 ]PF 6 PhMe 2 SiH acetone 71% OTIPS H O PhMe 2 Si OAc
Key Oxidative Cyclization OTIPS OTIPS DDQ, LiClO 4 H O O 2,6-Cl 2 Py PhMe 2 Si PhMe 2 Si DCE, 40 °C, 6 h 51% OAc O OTIPS OTIPS DDQ, LiClO 4 H 6 steps AcO O AcO O 2,6-Cl 2 Py clavosolide A 14 steps (LLS), 20 total steps DCE, 1.5 h 62% OAc O
Rychnovsky Retrosynthesis of Cyanolide A Sakurai dimerization/ macrocyclization TMS H O O O OMe MeO O OH O O O OMe O O MeO O O O OMe OH O OMe O O O H TMS O OH esterfication O OH TMS HO TMS O OH CH(OMe) 2 CH(OMe) 2 Gesinki, M.R.; Rychonovsky, S.D. J. Am. Chem. Soc. 2011 , 133 , 9727.
Rychnovsky “Monomer” Synthesis 1. ClMgCH 3 TMS 1. LiCH 2 TMS Pd(PPh 3 ) 4 OEt O OEt 97% 87% EtO OEt 2. KHMDS, PhNTf 2 EtO OTf 2. p TSA · H 2 O 97% 97% S 1. (-)-sparteine O PhBCl 2 O O OH S N 70% + TMS TMS H HO 2. LiOH 91% OH I 2 , MeOH O (MeS) 2 CH 2 CH(SMe) 2 62% n -BuLi 100% O OH OH TMS O OH Cl 3 PhCOCl O CH(OMe) 2 + TMS HO DMAP, Et 3 N 61% CH(OMe) 2
Rychnovsky Formal Total Synthesis H H O OH O O O TMS O O O O OsO 4 , NMO; O TMSOTf O O O O NaIO 4 CH 2 Cl 2 82% 76% O H O O H CH(OMe) 2 H OH O Ref. O O NaBH 4 cyanolide A O O 96% HO O H
Krische First Generation Synthesis MesN NMes Cl Ru Cl COEt [{Ir(cod)Cl} 2 ] (5 mol%) i PrO chiral ligand (10 mol%) OH OH OH OH (10 mol%) 4-Cl-3-NO 2 -BzOH (20 mol%) OH Cs 2 CO 3 (40 mol%) O O (2000 mol%) dioxane, 100 ° C AcO EtOC (s)-Cl,MeO-biphep (1000 mol%) DCE, 80 ° C 48%, 20:1 d.r., >99% ee 75%, 10:1 d.r. (S)-binap (gram scale) 40%, 20:1 d.r., >99% ee MesN NMes Cl OMe Ru Cl PhS OMe OMe i PrO O OH O OMe OMe (10 mol%) (150 mol%) O O O ethylene (1 atm) MeOTf (40 mol%) OMe toluene, 60 ° C 4 Å M.S. (200 wt%) EtOC EtOC Et 2 O 70% 69%, 2:1 d.r. Waldeck, A.R.; Krische, M.J. Angew. Chem. Int. Ed. 2013 , 52 , 1. Iridium-Catalyzed Carbonyl Allylation: Kim. I.S.; Ngai, M.Y.; Krische, M.J. J. Am. Chem. Soc. 2008 , 130 , 14891.
Krische First Generation Synthesis OMe OMe O OMe OsO 4 (1 mol%) O OMe Li( s Bu) 3 BH (100 mol%) oxone (400 mol) O O OMe O O THF, -78 to 25 ° C OMe DMF HO EtOC 73%, 4:1 d.r. CO 2 H OMe O O O OMe OMe MeO MNBA (300 mol%) O O O OMe DMAP (2000 mol%) O O OMe MeO O O O toluene, 90 ° C OMe HO 18 % (over 2 steps) OMe O O cyanolide A 7 steps (LLS), 11 total steps
Krische Second Generation Synthesis OMe HO COEt PhS OMe COEt OMe O OMe O OMe ozone OMe (150 mol%) OH O OMe THF, -78 ° C; O O OMe Li( s Bu) 3 BH (1000 %) MeOTf (40 mol%) O O O HO OMe 4 Å M.S. (200 wt%) THF, -78 to 25 ° C Et 2 O EtOC EtOC 68%, 2:1 d.r. 71%, 5:1 d.r. HO O OMe TEMPO (30 mol%) O OMe NaOCl (225 mol%), NaHCO 3(aq) 1,3,5,-Cl 3 -BzCl (120 mol%) CH 2 Cl 2 , 0 ° C; Et 3 N (140 mol%) cyanolide A O O OMe 6 steps (LLS), 10 total steps iosbutylene (1400 mol%), DMAP (500 mol%) HO toluene, 120 ° C NaClO 2 (225 mol%), Na 2 HPO 4(aq) , 47% NaBr (aq) , t BuOH 62%
Conclusions Ø Shortest route (6 steps LLS) to cyanolide A to date in 5.1% overall yield Ø Showcases an enantioselective Ir-catalyzed carbonyl allylation methodology utilizing alcohols Ø Demonstrates a Ru-catalyzed cross-metathesis/oxa-Michael cyclization
Recommend
More recommend