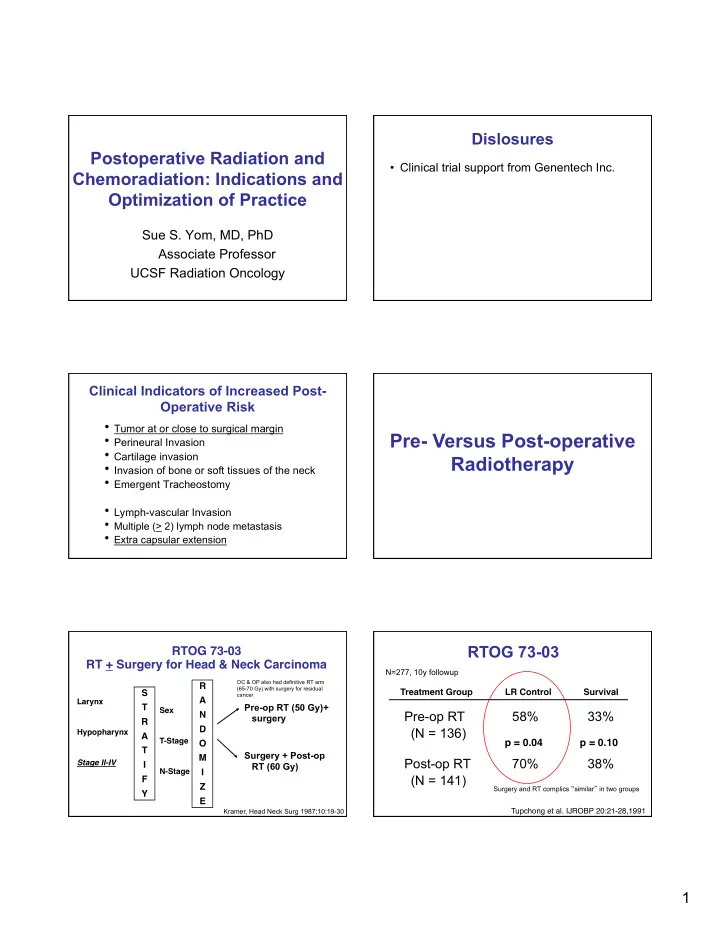

Dislosures Postoperative Radiation and • Clinical trial support from Genentech Inc. Chemoradiation: Indications and Optimization of Practice Sue S. Yom, MD, PhD Associate Professor UCSF Radiation Oncology Clinical Indicators of Increased Post- Operative Risk • Tumor at or close to surgical margin Pre- Versus Post-operative • Perineural Invasion • Cartilage invasion Radiotherapy • Invasion of bone or soft tissues of the neck • Emergent Tracheostomy • Lymph-vascular Invasion • Multiple (> 2) lymph node metastasis • Extra capsular extension RTOG 73-03 RTOG 73-03 � RT + Surgery for Head & Neck Carcinoma � N=277, 10y followup OC & OP also had definitive RT arm R � (65-70 Gy) with surgery for residual Treatment Group � � LR Control � � Survival � S � cancer A � Larynx � Pre-op RT (50 Gy)+ T � Sex � Pre-op RT 58% 33% N � surgery R � D � (N = 136) Hypopharynx � A � T-Stage � p = 0.04 � p = 0.10 � O � T � Surgery + Post-op M � Post-op RT 70% 38% Stage II-IV � � I RT (60 Gy) N-Stage � � I (N = 141) F � Z � Surgery and RT complics “ similar ” in two groups Y � E � Kramer, Head Neck Surg 1987;10:19-30 Tupchong et al. IJROBP 20:21-28,1991 � 1
Historical PORT results • Locoregional control 69-72% • 5-year survival 30-40% Radiation Dose MD Anderson randomized dose-finding MDA dose finding results study N=240 • <54 Gy had significantly higher failure rate R � Stratification � Low risk -> no radiation • ECE needed at least 63 Gy A � • 2-3 negative factors increased LR recurrence risk: • Oral Cavity � N � Dose A 57 Gy/32 Fx – oral cavity D � Int Risk* – close/pos margins • Larynx � O � Dose B 63 Gy/35 Fx – perineural M � – >2 involved nodes High Risk • Hypopharynx � � I – node >3 cm Dose C 68.4 Gy/38 Fx – treatment delay >6 wks Z � • Larynx � – Zubrod performance status>2 E � • Based on T- & N-stage, margin, PNI • Raised midway from 52.2-54 Gy/29-30 Fx 4 negative factors à à locoregional recurrence risk similar to ECE Radiation Timing Peters, IJROBP, 1993, 26:3-11 2
MD Anderson study on accelerated RT oral cavity, oropharynx, larynx, LRC & OS by hypopharynx package time (date of surgery to PORT completion) – Last 2 wks CCB for high risk pts Pathologic T stage was T3–4 in 129 (61%) and N2–3 in 123 (58%) patients Ang KK, IJROBP 2001, 51:571 LRC and OS based on interval from surgery to PORT median = 31d Chemotherapy Randomized trials of RT vs chemoRT: Rationale for chemoradiation EORTC 22931 & RTOG 9501 • To overcome radioresistance • To increase local control • To eradicate systemic micro mets • To counteract accelerated repopulation after surgical cytoreduction NEJM 2004; 350:1945-1952 NEJM 2004: 350:1937-1944 3
EORTC vs RTOG – LRC 11-13% EORTC vs RTOG – OS 10-11% improvement improvement EORTC RTOG EORTC RTOG NOT STATISTICALLY SIGNIFICANT EORTC & RTOG - Combined data RTOG 9501: 10 year followup • No overall benefit for LRC or OS from postop chemoradiation at 10 years – LRC still better for ECE or pos margins 30% • Multiple nodes without ECE or pos margin: reduction in risk of shows no LRC benefit from postop CRT death – Analysis of patients with up to 6 involved nodes • Conclusion: Multiple nodes is not an indicator for postoperative chemoradiation – Suggestion of unexplained non-cancer related deaths in patients who received chemo in absence of ECE/+marg RTOG 0920 for intermediate (NOT HIGH RISK) cancers OC, larynx, OPX p16+/- R � RT: 60 Gy in 30 fractions Other ideas: Using A � Intermediate risk N � factors: cT2-3, N0-2 Targeted Therapy D � (minimal T4a) RT: 60 Gy in 30 fractions O � Stage III-IVA Cetuximab 400 mg/m2 loading, PNI M � 250 mg/m2 x 10 cycles LVSI I � Close <5mm Z � >5mm deep Open and accruing, goal is 700 pts E � 4
Postop chemoradiation + targeted Historically based comparison: DFS therapy: phase II RTOG 0234 for high for RT-Doc/cetuximab vs RTOG 9501 risk disease 100 100 / Disease-Free Survival (%) Disease-Free Survival (%) Led to / / 75 75 0234 RT+Doc+Cet creation of / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / cetuximab / RTOG 1216: / / / / / / HR (95% CI) / / 50 50 / / / open trial of 0.72 (0.50, 1.02) 9501 RT+CDDP 1-sided log-rank p=0.031 cisplatin vs 25 25 docetaxel vs doc+cetux cetuximab 0 0 0 0 1 1 2 2 3 3 Years after Registration Years after Registration Patients at Risk RTOG 0234 106 82 56 14 RTOG 9501 202 131 109 90 Special case? Extracapsular Conclusions extension in HPV resected dz • Postoperative not preoperative radiation is standard. • Reporting of ECE in practice is not graded/detailed • Accelerated fractionation may benefit patients with a • Clinical guidelines and randomized study data do not delayed RT start. differentiate between different types of ECE • Total treatment package time is highly prognostic for high risk patients. • Patients with ≥ 2 LN, ECE, +margins are at the highest risk for recurrence. • 4+ clustered factors confer poor prognosis similar to ECE. • Postop chemo-RT is beneficial for patients with involved margins or ECE or both. Lewis et al, Modern Path 2011 • For p16+ oropharynx cancer, ECE may not carry negative • Postoperative therapy for HPV+ disease follows the standard of care for the moment but prognostic factors are prognosis until it reaches the level of soft tissue metastasis being re-analyzed. i.e. obliteration of nodal architecture (Sinha 2011) • Current trials incorporate targeted therapies; immune- – Caveat: based on Washington University retrospective review in based therapy is a future possibility. which half the patients received chemo-RT 5
Recommend
More recommend