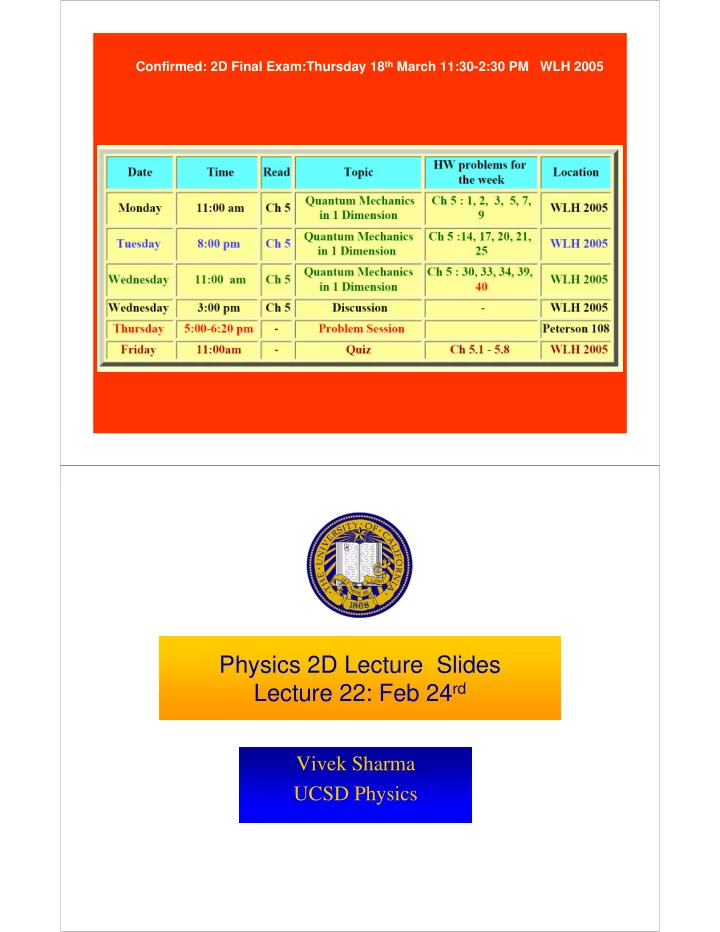

Confirmed: 2D Final Exam:Thursday 18 th March 11:30-2:30 PM WLH 2005 Physics 2D Lecture Slides Lecture 22: Feb 24 rd Vivek Sharma UCSD Physics
Introducing the Schrodinger Equation ∂ Ψ ∂Ψ � 2 2 ( , ) ( , ) x t x t − + Ψ = � ( ) ( , ) U x x t i ∂ ∂ 2 2 m x t • U(x) = characteristic Potential of the system • Different potential for different forces • Hence different solutions for the Diff. eqn. • � characteristic wavefunctions for a particular U(x) Schrodinger Wave Equation ψ Wavefunction which is a sol. of the Sch. Equation embodies all modern physics experienced/learnt so fa r: h ∆ ∆ ∆ ∆ ∼ � ∼ � E=hf, p= , x . p , E . t , quantiza tion etc λ Schrodinge r Equation is a D ynamical Equation � � much like Newton's Equation F = a m → ψ → → ψ (x,0) Force(potentia l ) (x,t) Evolves the System as a function of space-time The Schrodinger Eq. propogates the system forwar d & backward in time: ψ ⎡ ⎤ d ψ δ ψ ± ⎢ δ (x, t) = (x,0) t ⎥ ⎣ ⎦ dt = t 0 Where does it come from ?? ..."First Principles"..no real derivation exists
Time Independent Sch. Equation ∂ Ψ ∂Ψ � 2 2 ( , ) ( , ) x t x t − + Ψ = � ( ) ( , ) U x x t i ∂ ∂ 2 2 m x t Sometimes (depending on the character of the Potential U(x,t)) The Wave function is factorizable: can be broken up ( ) Ψ = ψ φ x,t ( ) ( ) x t Ψ ω = ω i(kx - t) i(kx) -i( t) : Plane Wave (x,t )=e e e Exa mple In suc h cases, use seperation of variables to get : ∂ ψ ∂ φ � 2 2 - ( ) x ( ) t φ + ψ φ = ψ � ( ). ( ) ( ) ( ) ( ) t U x x t i x ∂ ∂ 2 2m x t Ψ ψ φ Divide Throughout by (x,t )= ( x) ( t) ∂ ψ ∂ φ � 2 2 - 1 ( ) x 1 ( ) t ⇒ + = � . ( ) U x i ψ ∂ φ ∂ 2 2m ( ) ( ) x x t t L HS i s a function of x; RHS is fn of t x and t are independent variables, hence : ⇒ RHS = LHS = Constant = E Factorization Condition For Wave Function Leads to: ∂ ψ � 2 2 - ( ) x + ψ = ψ ( ) ( ) ( ) U x x E x ∂ 2 2m x ∂ φ ( ) t = φ � ( ) i E t ∂ t What is the Constant E ? How to Interpret it ? Back to a Free particle : ω Ψ ψ ikx -i t ikx (x,t)= Ae e , (x)= Ae U(x,t) = 0 ⇒ Plug it into the Time Independent Schrodinger Equation (TISE) − � 2 2 ( ) � 2 2 2 ikx ( ) d Ae k p + = ⇒ = = = ( ) ikx 0 (NR Energy) E A e E 2 2 2 2 m dx m m Ψ ψ ω -i t Stationary states of the free particle: (x,t)= (x)e 2 2 ⇒ Ψ = ψ ( , ) ( ) x t x ψ Probability is static in time t, character of wave function depends on ( ) x
Schrodinger Eqn: Stationary State Form • Recall � when potential does not depend on time explicitly – U(x,t) =U(x) only…we used separation of x,t variables to simplify • Ψ (x,t) = ψ (x) φ (t) • broke S. Eq. into two: one with x only and another with t only ∂ ψ � 2 2 - ( ) x + ψ = ψ ( ) ( ) ( ) U x x E x ∂ 2 2m x Ψ = ψ φ ( , ) ( ) ( ) x t x t ∂ φ ( ) t = φ � ( ) i E t ∂ t How to put Humpty-Dumpty back together ? e.g to say how to go from an expression of ψ (x) →Ψ (x,t) which describes time-evolution of the overall wave function Schrodinger Eqn: Stationary State Form d 1 d ( ) [ ] f t = Since ln ( ) f t dt ( ) dt f t ∂ φ ∂ φ ( ) 1 ( ) t t E iE = φ = = − � In i ( ) , rew rite as E t ∂ φ ∂ � � t ( ) t t i integrate both sides w.r.t. time and = ∂ φ φ t t t t 1 ( ) 1 d ( ) t iE t iE ∫ ∫ ∫ = − ⇒ = − dt dt dt φ ∂ φ � � ( ) t ( ) dt t t t=0 0 0 iE ∴ φ − φ = − ln ( ) ln (0) , n ow exponentiate both sides t t � iEt − ⇒ φ = φ φ = � ( ) (0) ; (0) constant= initial condition = 1 (e.g) t e iE i E t − − t ⇒ φ = Ψ ψ � � ( ) & T hus (x,t)= (x) where E = energy of system t e e
A More Interesting Potential : Particle In a Box Write the Form of Potential: Infinite Wall ∞ ≤ ≥ U(x) U(x,t) = ; x 0, x L U(x,t) = 0 ; 0 < X < L • Classical Picture: •Particle dances back and forth •Constant speed, const KE •Average <P> = 0 •No restriction on energy value • E=K+U = K+0 •Particle can not exist outside box •Can’t get out because needs to borrow infinite energy to overcome potential of wall What happens when the joker is subatomic in size ?? Example of a Particle Inside a Box With Infinite Potential (a) Electron placed between 2 set of electrodes C & grids G experiences no force in the region between grids, which are held at Ground Potential However in the regions between each C & G is a repelling electric field whose strength depends on the magnitude of V (b) If V is small, then electron’s potential energy vs x has low sloping “walls” (c) If V is large, the “walls”become very high & steep becoming infinitely high for V →∞ (d) The straight infinite walls are an approximation of such a situation U= ∞ U(x) U= ∞
Ψ (x) for Particle Inside 1D Box with Infinite Potential Walls ⇒ Inside the box, no force U=0 or constant (same thing) Why can’t the ψ � 2 2 - d ( ) x ⇒ + ψ = ψ 0 ( ) ( ) x E x particle exist 2 2m dx ψ 2 ( ) 2 d x mE Outside the box ? ⇒ = − ψ = 2 2 ( ) ; k x k 2 � 2 dx � E Conservation ψ 2 d ( ) x + ψ = ⇐ ψ 2 ( ) 0 fig ure out what (x) solves this diff e q. or k x ∞ ∞ 2 dx ψ = + In General the solu t io n is ( ) (A,B are constants) x A sinkx B coskx Need to figure out values of A, B : How to do that ? A p pl y BO UNDA R Y Conditions on the Physical Wav efunction ψ We said ( ) must be continuous everywhe re x So match the wavefunction just outside box to the wavefunction value just inside the box ⇒ ⇒ ψ = = ⇒ ψ = = At x = 0 ( 0) 0 & At x = L ( ) 0 x x L ∴ ψ = = = ( 0) 0 (Continuity condition at x =0) x B ψ = = ⇒ X=L & ( ) 0 A Sin kL = 0 (Continuity condition at x =L) X=0 x L π n ⇒ π ⇒ = ∞ kL = n k = , n 1,2,3,... L π 2 2 � 2 n So what does this say about Energy E ? : E = Quantized (not Continuous)! n 2 2 mL Quantized Energy levels of Particle in a Box
What About the Wave Function Normalization ? The particle's Energy and Wavefu nct ion a re determi ned by a nu mb er n → We will call n Quantum Number , just like in Bohr's Hydrogen atom W hat about the wave functions cor res pondi ng to each of these e ner g y states? π n x ψ = = sin( ) sin( ) for 0<x < L A kx A n L ≥ ≥ = 0 for x 0, x L Normalized Condition : L π L n x ∫ ∫ ψ ψ = θ = − θ * 2 2 2 1 = ( ) Use 2Sin 1 2 2 dx A S in Cos n n L 0 0 π 2 L ⎛ ⎞ 2 A n x ∫ ∫ = − θ θ 1 ⎜ 1 c os( ) and since cos = sin ⎟ ⎝ ⎠ 2 L 0 2 2 A L = ⇒ = 1 A 2 L π 2 2 n x ψ = = So sin( ) sin ( ) ...What does this look l ike? kx n L L L Wave Functions : Shapes Depend on Quantum # n Probability P(x): Where the Wave Function particle likely to be Zero Prob
Where in The World is Carmen San Diego? • We can only guess the probability of finding the particle somewhere in x – For n=1 (ground state) particle most likely at x = L/2 – For n=2 (first excited state) particle most likely at L/4, 3L/4 • Prob. Vanishes at x = L/2 & L – How does the particle get from just before x=L/2 to just after? » QUIT thinking this way, particles don’t Classically, where is particle most likely to be ? have trajectories Equal prob. of being anywhere inside the Box » Just probabilities NOT SO says Quantum Mechanics! of being somewhere Remember Sesame Street ? This particle in the box is brought to you by the letter Its the Big Boss Quantum Number
How to Calculate the QM prob of Finding Particle in Some region in Space Consider n =1 state of the particle L 3 L ≤ ≤ Ask : What is P ( )? x 4 4 3 3 3 L L L π π ⎛ ⎞ 4 4 4 2 2 1 2 x x ∫ ∫ ∫ ψ 2 = = − 2 P = sin ⎜ ⎟ . (1 cos ) dx dx dx 1 ⎝ ⎠ 2 L L L L L L L 4 4 4 π 3 /4 L π π ⎡ ⎤ ⎡ ⎤ ⎛ ⎞ 1 2 1 1 2 3 2 L L x L L = − = − − sin ⎜ sin . sin . ⎟ P ⎢ ⎥ ⎢ ⎥ π π ⎣ ⎦ ⎣ ⎦ ⎝ ⎠ 2 2 2 2 4 4 L L L L /4 L 1 1 ( 1 1) = − − − = ⇒ 0.818 8 1. 8 % P 2 π 2 ⇒ Classically 50% (equal prob over half the box size) ⇒ Substantial difference between Class ical & Quantu m predictio n s When The Classical & Quantum Pictures Merge: n →∞ But one issue is irreconcilable: Quantum Mechanically the particle can not have E = 0 This is a consequence of the Uncertainty Principle The particle moves around with KE inversely proportional to the Length Of the 1D Box
Recommend
More recommend