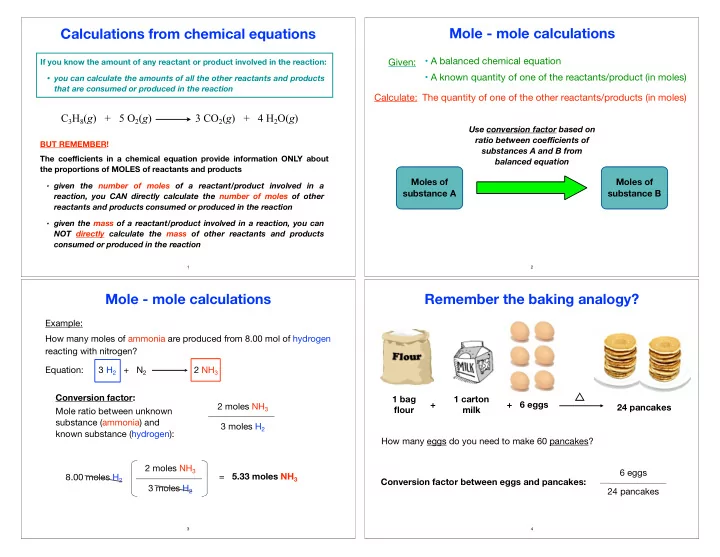

Mole - mole calculations Calculations from chemical equations • A balanced chemical equation Given: If you know the amount of any reactant or product involved in the reaction: • A known quantity of one of the reactants/product (in moles) • you can calculate the amounts of all the other reactants and products that are consumed or produced in the reaction Calculate: The quantity of one of the other reactants/products (in moles) C 3 H 8 ( g ) + 5 O 2 ( g ) 3 CO 2 ( g ) + 4 H 2 O( g ) Use conversion factor based on ratio between coe ffi cients of BUT REMEMBER! substances A and B from The coe ffi cients in a chemical equation provide information ONLY about balanced equation the proportions of MOLES of reactants and products Moles of Moles of • given the number of moles of a reactant/product involved in a substance A substance B reaction, you CAN directly calculate the number of moles of other reactants and products consumed or produced in the reaction • given the mass of a reactant/product involved in a reaction, you can NOT directly calculate the mass of other reactants and products consumed or produced in the reaction 1 2 Mole - mole calculations Remember the baking analogy? Example: How many moles of ammonia are produced from 8.00 mol of hydrogen reacting with nitrogen? Equation: 3 H 2 + N 2 2 NH 3 Conversion factor: 1 bag 1 carton + + 6 eggs 2 moles NH 3 24 pancakes flour milk Mole ratio between unknown substance (ammonia) and 3 moles H 2 known substance (hydrogen): How many eggs do you need to make 60 pancakes? 2 moles NH 3 6 eggs = 5.33 moles NH 3 8.00 moles H 2 Conversion factor between eggs and pancakes: 3 moles H 2 24 pancakes 3 4
Remember the baking analogy? Mole - mole calculations Given the balanced equation: K 2 Cr 2 O 7 + 6 KI + 7 H 2 SO 4 Cr 2 (SO 4 ) 3 + 4 K 2 SO 4 + 3 I 2 + 7 H 2 O a) How many moles of potassium dichromate (K 2 Cr 2 O 7 ) are required to react with 2.0 mol of potassium iodide (KI) 1 bag 1 carton + + 6 eggs 24 pancakes Conversion Factor: flour milk 1 mol K 2 Cr 2 O 7 Mole ratio between the unknown substance (potassium dichromate) and 6 mol Kl How many eggs do you need to make 60 pancakes? the known substance (potassium iodide): 6 eggs 1 mol K 2 Cr 2 O 7 = 15 eggs 60 pancakes = 0.33 mol K 2 Cr 2 O 7 2.0 mol KI 24 pancakes 6 mol Kl 5 6 Mole - mass calculations Mole - mole calculations Given: • A balanced chemical equation Given the balanced equation: • A known quantity of one of the reactants/product (in moles) K 2 Cr 2 O 7 + 6 KI + 7 H 2 SO 4 Cr 2 (SO 4 ) 3 + 4 K 2 SO 4 + 3 I 2 + 7 H 2 O Calculate: The mass of one of the other reactants/products (in grams) b) How many moles of sulfuric acid (H 2 SO 4 ) are required to produce 2.0 moles of iodine (I 2 ) Grams of substance B Conversion factor: 7 mol H 2 SO 4 Mole ratio between the unknown substance (sulfuric acid) and the 3 mol l 2 known substance (iodine): Use molar mass of substance B Use ratio between coe ffi cients of substances A and B from balanced equation 7 mol H 2 SO 4 2.0 mol l 2 = 4.7 mol H 2 SO 4 Moles of Moles of 3 mol l 2 substance A substance B 7 8
Mass - mole calculations Mole - mass calculations Given: • A balanced chemical equation Example: • A known mass of one of the reactants/product (in grams) What mass of hydrogen is produced by reacting 6.0 mol of aluminum with hydrochloric acid? Calculate: The quantity of one of the other reactants/products (in moles) Equation: 2 Al ( s ) + 6 HCl ( aq ) 2 AlCl 3 ( aq ) + 3 H 2 ( g ) Grams of Conversion Factor: substance A 3 mol H 2 Mole ratio between unknown substance (hydrogen) and 2 mol Al Use molar mass known substance (aluminum): of substance A Use ratio between coe ffi cients of substances A and B from 3 mol H 2 2.016 g balanced equation = 18 g H 2 6.0 mol Al = 9.0 mol H 2 Moles of Moles of 1 mol H 2 2 mol Al substance A substance B 9 10 Mass - mole calculations Mass - mole calculations How many moles of silver nitrate (AgNO 3 ) are required to produce How many moles of silver nitrate (AgNO 3 ) are required to produce 100.0 g of silver sulfide (Ag 2 S)? 100.0 g of silver sulfide (Ag 2 S)? 2 AgNO 3 + H 2 S Ag 2 S + 2 HNO 3 ! 2 AgNO 3 + H 2 S Ag 2 S + 2 HNO 3 Step 2: Determine the number of moles of the unknown substance (AgNO 3 ) required to produce the number of moles of the known Step 1: Convert the amount of known substance (Ag 2 S) from substance (0.403 mol Ag 2 S) grams to moles Conversion Factor: 2 mol AgNO 3 Mole ratio between the unknown 1 mol Ag 2 S substance (silver nitrate) and the 100.0 g Ag 2 S = 0.403 mol Ag 2 S 1 mol Ag 2 S known substance (silver sulfide): 247.87 g Ag 2 S 2 mol AgNO 3 0.403 mol Ag 2 S = 0.806 mol AgNO 3 1 mol Ag 2 S 11 12
Mass - mass calculations Mass - mass calculations Given: • A balanced chemical equation How many grams of nitric acid are required to produce 8.75 g of • A known mass of one of the reactants/product (in grams) dinitrogen monoxide (N 2 O)? Calculate: The mass of one of the other reactants/products (in grams) The balanced equation is: 4 Zn ( s ) + 10 HNO 3 ( aq ) 4 Zn(NO 3 ) 2 ( aq ) + N 2 O ( g ) + 5 H 2 O ( l ) Grams of Grams of Step 1: Convert the amount of known substance (N 2 O) from substance A substance B grams to moles Use molar mass Molar mass N 2 O: ( 2 x 14.01 g/mol ) + 16.00 g/mol = 44.02 g/mol Use molar mass of substance A of substance B Use ratio between coe ffi cients of substances A and B from 1 mol N 2 O balanced equation 8.75 g N 2 O = 0.199 mol N 2 O Moles of Moles of 44.02 g N 2 O substance A substance B 13 14 Mass - mass calculations Mass - mass calculations How many grams of nitric acid are required to produce 8.75 g of How many grams of nitric acid are required to produce 8.75 g of dinitrogen monoxide (N 2 O)? dinitrogen monoxide (N 2 O)? The balanced equation is: The balanced equation is: 4 Zn ( s ) + 10 HNO 3 ( aq ) 4 Zn(NO 3 ) 2 ( aq ) + N 2 O ( g ) + 5 H 2 O ( l ) 4 Zn ( s ) + 10 HNO 3 ( aq ) 4 Zn(NO 3 ) 2 ( aq ) + N 2 O ( g ) + 5 H 2 O ( l ) Step 2: Determine the number of moles of the unknown substance (HNO 3 ) required to Step 3: Convert the amount of unknown substance (1.99 moles HNO 3 ) produce the number of moles of the known substance (0.199 mol N 2 O) from moles to grams Conversion Factor: 10 mol HNO 3 Mole ratio between the unknown Molar mass HNO 3 : 1.008 g/mol + 14.01 g/mol + ( 3 x 16.00 g/mol ) substance (nitric acid) and the known 1 mol N 2 O = 63.02 g/mol substance (dinitrogen monoxide): 10 mol HNO 3 63.02 g HNO 3 = 1.99 mol HNO 3 0.199 mol N 2 O = 125 g HNO 3 1.99 mol HNO 3 1 mol N 2 O 1 mol HNO 3 15 16
Mass - mass calculation: Another example Mass - mass calculation: Another example How many grams of carbon dioxide are produced by the complete How many grams of carbon dioxide are produced by the complete combustion of 100. g of pentane (C 5 H 12 )? combustion of 100. g of pentane (C 5 H 12 )? The balanced equation is: The balanced equation is: C 5 H 12 ( g ) + 8 O 2 ( g ) 5 CO 2 ( g ) + 6 H 2 O( g ) C 5 H 12 ( g ) + 8 O 2 ( g ) 5 CO 2 ( g ) + 6 H 2 O( g ) Step 2: Determine the number of moles of the unknown substance (CO 2 ) required Step 1: Convert the amount of known substance (C 5 H 12 ) from to produce the number of moles of the known substance (1.39 mol C 5 H 12 ) grams to moles Conversion Factor: 5 mol CO 2 Molar mass C 5 H 12 : ( 5 x 12.01 g/mol ) + ( 12 x 1.008 g/mol ) Mole ratio between the unknown substance (carbon dioxide) and the = 72.15 g/mol 1 mol C 5 H 12 known substance (pentane): 1 mol C 5 H 12 5 mol CO 2 = 1.39 mol C 5 H 12 100. g C 5 H 12 = 6.95 mol CO 2 1.39 mol C 5 H 12 72.15 g C 5 H 12 1 mol C 5 H 12 17 18 Mass - mass calculation: Another example Yields How many grams of carbon dioxide are produced by the complete Theoretical yield -- the calculated amount (mass) of product that can combustion of 100. g of pentane (C 5 H 12 )? be obtained from a given amount of reactant based on the balanced chemical equation for a reaction The balanced equation is: Actual yield -- the amount of product actually obtained from a reaction C 5 H 12 ( g ) + 8 O 2 ( g ) 5 CO 2 ( g ) + 6 H 2 O( g ) The actual yield observed for a reaction is almost always less than the theoretical yield due to: Step 3: Convert the amount of unknown substance ( 6.95 moles CO 2 ) • side reactions that form other products from moles to grams • incomplete / reversible reactions • loss of material during handling and transfer from one vessel to another Molar mass CO 2 : 12.01 g/mol + ( 2 x 16.00 g/mol ) = 44.01 g/mol The actual yield should never be greater than the theoretical yield — if it is, it is an indicator of experimental error 44.01 g CO 2 6.95 mol CO 2 = 306 g CO 2 actual yield 1 mol CO 2 Percent yield = 100 x theoretical yield 19 20
Recommend
More recommend