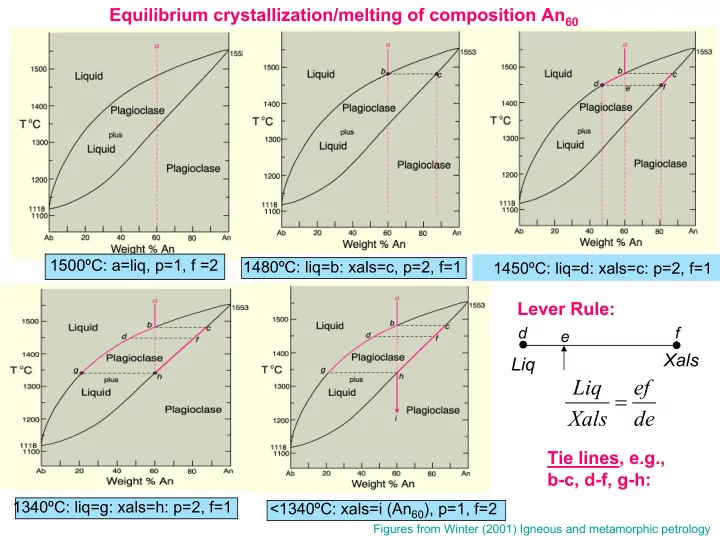

Equilibrium crystallization/melting of composition An 60 1500ºC: a=liq, p=1, f =2 1480ºC: liq=b: xals=c, p=2, f=1 1450ºC: liq=d: xals=c: p=2, f=1 Lever Rule: d f e Xals Liq Liq = ef Xals de Tie lines, e.g., b-c, d-f, g-h: 1340ºC: liq=g: xals=h: p=2, f=1 <1340ºC: xals=i (An 60 ), p=1, f=2 Figures from Winter (2001) Igneous and metamorphic petrology
Fractional crystallization/melting of composition An 60 With fractional melting of comp n i, the first With fractional crystallization of bulk comp n a , first crystals have comp n c . Crystals are liquid to form will have comp n g . Each removed from system as soon as they form fraction of melt produced is removed from so are not present to react with liquid. The the system so the solid assemblage changes comp n from h to pure An and liquid will fractionate to pure albite comp n and the total range of plagioclase crystals the liquid fractions range from g to pure An . Mean comp n of liquid is a will be from c to pure Ab . Figures from Winter (2001) Igneous and metamorphic petrology
Binary systems with an intermediate compound 1800 b 1600 L c d Cr + L T(ºC) d a b c 1400 Meta- stable Tr + L 1200 Ab + L Ne + L 1118 1068 1062 1000 Ab + Tr Ne + Ab 0 20 40 60 80 100 SiO 2 Ab Ne Peritectic system: intermediate “Double eutectic” system : Intermediate compound (En) melts incongruently compound (Ab) melts congruently En ↔ Fo + Liq Ab ↔ Liq Melting reaction: Melting reaction: Discuss: Isobaric univariant and invariant equilibria Discuss: Equilibrium/fractional crystallization/melting Figure from Winter (2001) Igneous and metamorphic petrology
Fo – SiO 2 system (cont.) i i k k m m 1557 1557 d d c c Fo Fo En En Effect of pressure on the Fo + En What happens in the vicinity + Liquid equilibrium of the peritectic point? 1. Liquidus and solidus temperatures increase Cooling: at 1557ºC, Liquid i reacts with Fo to form En 2. Peritectic reaction becomes a eutectic reaction Heating: At 1557ºC, En crystals Does this have any application in nature? melt to give Fo + liquid i Figures from Winter (2001) Igneous and metamorphic petrology
A. Photomicrograph of olivine with a rim of Orthopyroxene (crossed polars) This is a common petrographic feature of basalts crystallized under low pressure but it may not occur at higher B. Same grain with slide rotated until pressures olivine is at extinction position to make the opx rim more visible
Binary systems (cont.) : Ab (NaAlSi 3 O 8 )-Or (KAlSi 3 O 8 ): Example of an azeotropic system with minimum in the solidus and liquidus and a solvus P = 1 atm Note: a L 1. Minimum in liquidus and solidus metastable L + Af ss loops at X m = 0.34. Common horiz 1100 tangent to both lines at this point. X m = 0.34 2. Region marked “Af ss ” shows complete solid solution from Or to Ab. Do we see this in nature? Af ss 900 3. In region underneath solvus two feldspars coexist, each showing T(ºC) limited solid solution. 4. As temperature decreases, the X c = 0.33 two feldspars become increasingly 700 separated in composition. 5. At the liquidus the Or-rich part of the diagram is metastable Ab ss + Or ss because Orthoclase melts incongruently to Leucite 500 6. What are perthites? 20 Ab 40 60 80 Or Consider cooling of composition a Wt % Or
Ab-Or system at 5 kilobars with excess H 2 O, i.e, P H2O = 5 kb Note: 1. This is actually a 3 component 900 a system. Why? L + V H 2 O is also a component along with Ab and Or. This figure is a 800 projection of the V-saturated Sa+L+V Ab+L+V equilibria from H 2 O. 2. What are the phases that coexist at 701 700 701ºC? What is the variance of the Ab ss +V Sa ss +V system at this temperature? Phases: Ab ss , Sa ss , L, V Ab ss +Sa ss +V f = 3-4+1 =0 (Isobaric invariant) 600 3. Why is the eutectic temperature much lower than in the “dry” system? 500 Effect of H 2 O on silica-rich melts 20 40 80 60 Or Ab is pronounced Wt % Or 4. How does the equilibria in Discuss the cooling of composition a from this system help to explain 2- above the V-saturated liquidus (T>800ºC). feldspar granites. After Morse (1970) Journal of Petrology
c (001) a perthite TEM image of albite lamellae (showing polysynthetic twinning) in a K-feldspar host (100) cleavage Plane // (010) Sketch showing the orientation of perthitic lamellae of albite in a K- feldspar host Optical photomicrograph of wispy albite lamellae in a microcline host
Recommend
More recommend