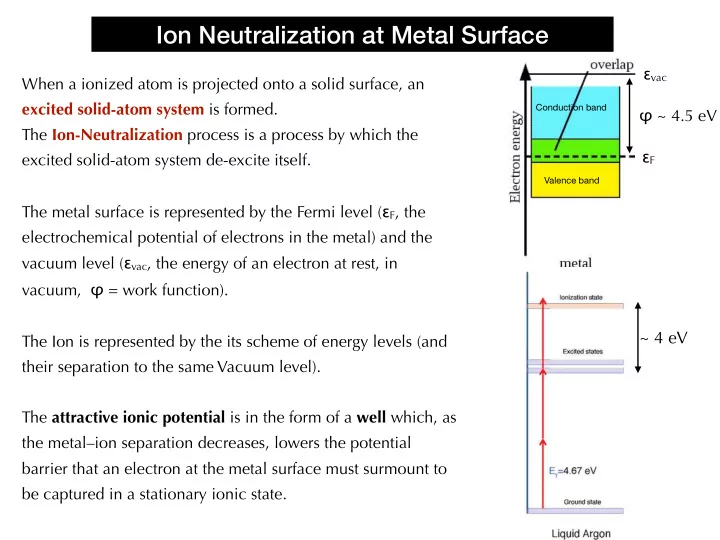

Ion Neutralization at Metal Surface ε vac When a ionized atom is projected onto a solid surface, an excited solid-atom system is formed. Conduction band φ ~ 4.5 eV The Ion-Neutralization process is a process by which the ε F excited solid-atom system de-excite itself. Valence band The metal surface is represented by the Fermi level ( ε F , the electrochemical potential of electrons in the metal) and the vacuum level ( ε vac , the energy of an electron at rest, in vacuum, φ = work function). ~ 4 eV The Ion is represented by the its scheme of energy levels (and their separation to the same Vacuum level). The attractive ionic potential is in the form of a well which, as the metal–ion separation decreases, lowers the potential barrier that an electron at the metal surface must surmount to be captured in a stationary ionic state.
Vacant ionic states are represented by open circles, occupied surface states by filled circles. Different neutralization (de-excitation) mechanisms can take place: - “one-electron process” : an electron in the metal band tunnels out from the surface to an excited state of the ion that is energetically degenerate with the surface state - This is called Resonance Neutralization [Fig.(a)] . - “two-electron process” : one electron in the metal band tunnels out from the surface to a more tightly bound state of the Ion. Energy is conserved by the emission of a second (Auger) electron [Fig.(b) - called Auger Neutralization - AN ] or a photon [Fig.(c)]. The Auger electron is in the metal surface and can be excited above the vacuum level if the energy balance is positive. Journal of Microscopy, Vol. 205, Pt 1 January 2002, pp. 86–95 - Auger Neutralization - AN - Resonance Neutralization For singly charged ions the Auger electron emission is more probable than photon emission because the Auger transition lifetime is about 10 6 times shorter than the radiative lifetime of approximately 10 –8 s. Being two-electron processes, Auger processes are generally less e ffi cient than resonant charge transfer - however Auger processes may be the dominating process where the Resonant Process are energetically forbidden.
MicroBooNE Case with a c.r. flux at surface of ~9kHz and an avg. track length of 2.1 m, ≈ 1.5x10 9 e-Ion pairs/m 3 s are generated Ions drift to the cathode, and the Ion current j+ impinging upon the metal surface (x=d) is j+ = n+(x=d) * v d + ≈ 4x10 9 I+/m 2 s The Tot. Ion flow rate at the Cathode Surface (S c =25 m 2 ) is j+ * S c ≈ 10 11 I+/s The (measured) average number of AN electrons emitted per Ar+ ion is 𝜹 i (Ar+) = 10 -1 e/I+ ⟹ AN Electron emission rate: (Ion Flow Rate) * 𝜹 i = 10 10 e/s = 𝒫 (few nA) [and negligible Photon emission rate] arXiv:1703.10491 [physics.ins-det]
Cosmic Evt.s form ICARUS Run at Surface Jun. 2001 (rare) Cosmic events may produce a blast of ionization in the TPC Volume - factor 10 3 or more wrt avg. cosmic ionization ⟹ AN mechanism may potentially induce outburst current of ~few μ A delayed 𝒫 (min) of time - when the Ions reach the Cathode
Caveat 1: the AN mechanism is well known in the case of atomic Ions (e.g. Ar+). In Liquid Argon Ar+ ions quickly convert into Ar2+ ionic dimers. The AN mechanism is known to hold as well for ionic dimers but is less efficient than for Ions. To be noted: no experimental data were found available for AN processes with Ar2+ Caveat 2: in case of insulating surface (resistive Cathode) - instead of metal surface - the situation is more complex as additional energy dissipation channels are possible. Whether AN remains the dominant neutralization channel, … we don’t know (no data are reported in literature).
Recommend
More recommend