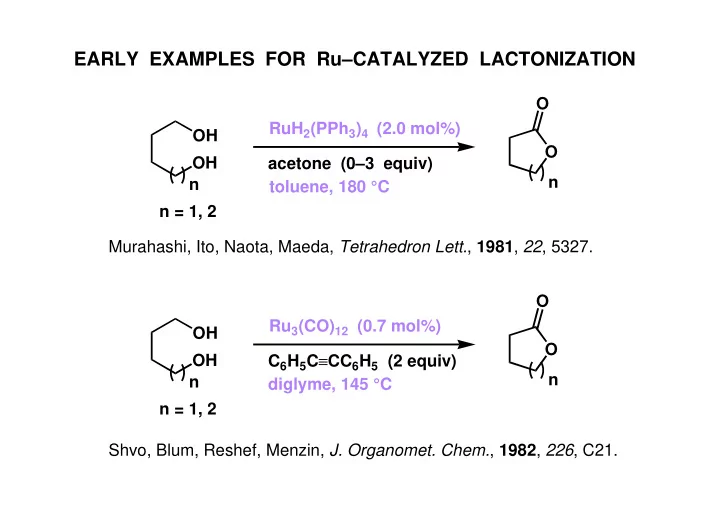

EARLY EXAMPLES FOR Ru–CATALYZED LACTONIZATION O RuH 2 (PPh 3 ) 4 (2.0 mol%) OH O OH acetone (0–3 equiv) n n toluene, 180 °C n = 1, 2 Murahashi, Ito, Naota, Maeda, Tetrahedron Lett. , 1981 , 22 , 5327. O Ru 3 (CO) 12 (0.7 mol%) OH O C 6 H 5 C ≡ CC 6 H 5 (2 equiv) OH n n diglyme, 145 °C n = 1, 2 Shvo, Blum, Reshef, Menzin, J. Organomet. Chem. , 1982 , 226 , C21.
NEW CATALYSTS FOR DEHYDROGENATIVE OXIDATION OF sec -ALCOHOL Ar Ar Cp*RuCl(cod) P ligand OH OH H Ru KO t -Bu N H C 6 H 5 C 6 H 5 R' toluene, 30 °C C H O R alcohol:Ru:ligand:KO t -Bu = 100:1:1:1 possible transition state ligand: N(CH 3 ) 2 (C 6 H 5 ) 2 P NH 2 (C 6 H 5 ) 2 P NHCH 3 (C 6 H 5 ) 2 P 476 TOF/h –1 188 <1 (H 3 C) 2 N NH 2 (C 6 H 5 ) 2 P P(C 6 H 5 ) 2 C 6 H 5 S NH 2 14 <1 <1
LIGAND ACCELERATION IN LACTONIZATION OF DIOL Cp*RuCl(cod) O ligand KO t -Bu OH O acetone OH 30 °C, 1 h diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M ligand: N(CH 3 ) 2 (C 6 H 5 ) 2 P NH 2 (C 6 H 5 ) 2 P NHCH 3 (C 6 H 5 ) 2 P >99% conv >99% 5% (C 6 H 5 ) 2 P P(C 6 H 5 ) 2 RuH 2 [P(C 6 H 5 ) 4 ] Ru 3 (CO) 12 17% 0% 0%
A RAPID LACTONIZATION OF 1,2-BENZENEDIMETHANOL Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O OH acetone, 30 °C 575 TOF/h -1 diol:Ru:ligand:KO t -Bu = 500:1:1:1, [diol] = 0.5 M Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O OH acetone, 30 °C CH 3 D (0.96D) CH 3 D (0.96D) Cp*RuCl(cod) (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 O KO t -Bu N. R. O acetone, 30 °C
A POSSIBLE MECHANISM O Ru Ar OH P H Ar H N OH 2 OH O Cp*RuCl(cod) + OH Ru Ru OH (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 Ar Ar P P HN HN + O Ar Ar KO t -Bu OH O Ru Ar P H O Ar O H N 2
LACTONIZATION OF UNSYMMETRICAL DIOLs Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O OH acetone, 30 °C, 1 h R 1 R 2 R 1 R 2 diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M R 1 R 2 yield, % CH 3 H >99 CH 3 D (96%) >99 (96% atom D) CH 3 CH 3 >99
EXAMPLES OF VARIOUS 1,4–DIOLs OH OH OH OH OH OH OH OH >99% >99% >99% >99% yield OH OH OH OH OH OH >99% >99% >99% Conditions; diol:Cp*RuCl(cod):(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 :KO t -Bu = 100:1:1:1 [diol] = 0.5 M in acetone, 30 °C, 1 h
EXAMPLES OF 1,5–DIOLs O Cp*RuCl(cod) (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH O KO t -Bu OH acetone, 30 °C, 1 h >99% yield Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O O + acetone, 30 °C, 1 h OH O 74 : 26 >99% yield diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M
EXAMPLES OF 1,6–DIOLs Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O acetone, 30 °C, 2 h OH diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M 97% yield Cp*RuCl(cod) (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu N. R. acetone, 30 °C, 1 h OH diol:Ru:ligand:KO t -Bu = 50:1:1.3:1.7, [diol] = 0.25 M
REGIOSELECTIVITY OF LACTONIZATION Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O + O OH acetone, 30 °C, 1 h O 95 : 5 >99% yield Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 OH KO t -Bu O O + OH acetone, 30 °C, 1 h O OCH 3 OCH 3 OCH 3 26 : 74 >99% yield diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M
FIRST REPORT ON Ru–CATALYZED ASYMMETRIC LACTONIZATION OH O Ru 2 Cl 4 ((-)diop) 3 (2.0 mol%) * R R O PhCH=CHCOCH 3 (2 equiv) toluene, reflux OH up to 15% ee O Ru 2 Cl 4 ((-)diop) 3 (2.0 mol%) OH O OH PhCH=CHCOCH 3 (2 equiv) n n toluene, reflux up to 12% ee Ishii, Osakada, Ikariya, Saburi, Yoshikawa, Chem Lett. , 1982 , 1179.
ENANTIOSELECTIVE LACTONIZATION OF meso -DIOL O Cp*RuCl(cod) chiral ligand OH KO t -Bu O OH acetone, 30 °C, 1 h (1 R , 2 S ) >99% yield diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M chiral ligand: (C 6 H 5 ) 2 P NH 2 (C 6 H 5 ) 2 P NH 2 CH(CH 3 ) 2 C 6 H 5 23% ee 18% ee H N (C 6 H 5 ) 2 P (C 6 H 5 ) 2 P NH 2 CH 2 C 6 H 5 7% ee 32% ee 44% ee (0°C, 8 h)
Recommend
More recommend