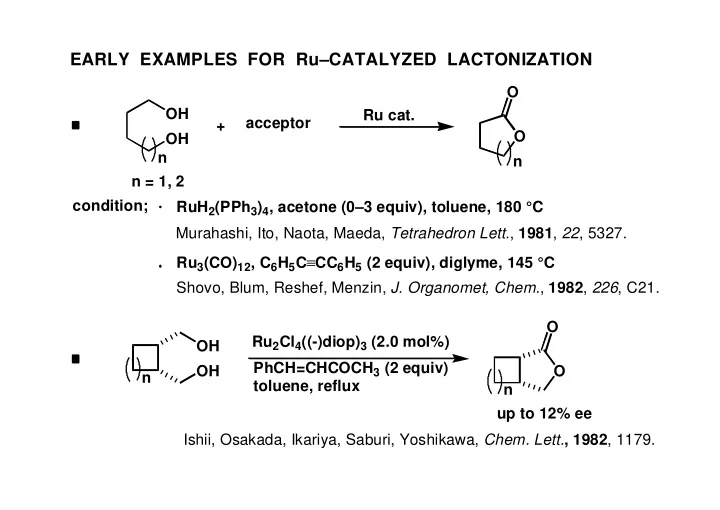

EARLY EXAMPLES FOR Ru–CATALYZED LACTONIZATION O OH Ru cat. acceptor + O OH n n n = 1, 2 condition; RuH 2 (PPh 3 ) 4 , acetone (0–3 equiv), toluene, 180 °C Murahashi, Ito, Naota, Maeda, Tetrahedron Lett. , 1981 , 22 , 5327. Ru 3 (CO) 12 , C 6 H 5 C ≡ CC 6 H 5 (2 equiv), diglyme, 145 °C Shovo, Blum, Reshef, Menzin, J. Organomet, Chem. , 1982 , 226 , C21. O Ru 2 Cl 4 ((-)diop) 3 (2.0 mol%) OH PhCH=CHCOCH 3 (2 equiv) OH O n toluene, reflux n up to 12% ee Ishii, Osakada, Ikariya, Saburi, Yoshikawa, Chem. Lett. , 1982 , 1179.
LACTONIZATION OF α , ω -DIOLS O Cp*RuCl(cod) R R OH (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 , KO t -Bu O OH acetone, 30 °C, 1 h R R >99% yield diol:Ru:ligand:KO t -Bu = 100:1:1:1, [diol] = 0.5 M 伊藤正人, 大迫章英, 碇屋隆雄, 第 7 9 春季年会, 1H 3 2 6 . a possible transformation Ar Ar Ar Ar P P H H Ru Ru N N H H R' R' C C H H O O R R
SYNTHESIS OF P–N LIGANDS O R 1 HN P(C 6 H 5 ) 2 CF 3 SO 3 H R 1 N + HP(C 6 H 5 ) 2 O S toluene R 2 S reflux, 24 h R 2 oxazolidinone:HP(C 6 H 5 ) 2 :CF 3 SO 3 H = 1:2:3 yield, % a R 1 R 2 [CH(CH 3 ) 2 ] 89 H i -Pr [CH 2 CH 2 (CH 3 ) 2 ] i -Bu 85 H [C(CH 3 ) 3 ] H t -Bu 66 [C 6 H 5 ] H Ph 70 [CH 2 C 6 H 5 ] H Bn 70 -(CH 2 ) 3 - 55 a Isolated yield
ENANTIOSELECTIVE LACTONIZATION chiral ligand Cp*RuCl(cod) chiral ligand S R 1 HN OH P(C 6 H 5 ) 2 KO t -Bu O OH acetone R R 2 30 °C, 1 h O diol:Ru:chiral ligand:KO t -Bu = 100:100:1:1:1 ee, % a R 1 , R 2 yield, % H, i -Pr >99 44 >99 42 H, i -Bu H, t -Bu >99 39 H, Bn 50 >99 >99 46 H, Ph -(CH 2 ) 3 - >99 10
ENANTIOSELECTIVE LACTONIZATION chiral ligand O Cp*RuCl(cod) R R H 2 N P(C 6 H 5 ) 2 chiral ligand * OH KO t -Bu O OH Bn 30 °C, 1 h * R R diol:Ru:chiral ligand:KO t -Bu = 100:100:1:1:1 R R R O O O O S S S O O O O 99% yield >99% yield >99% yield 99% yield 13% ee (1S,5R) 34% ee (1 R ,6 S ) 31% ee (3a R ,7a S ) 2% ee O O R R S S O O O O S S R R O O >99% yield >99% yield >99% yield >99% yield 16% ee (2 S ,3 R ) 11% ee (2 S ,3 R ) 43% ee (2 R ,3 S ) 50% ee (2R,3S)
CONTROL OF CONVERSION Cp*RuCl(cod) O (C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 O OH KO t -Bu + O OH toluene 30 °C, 1 h diol:Ru:ligand:KO t -Bu = 100:1:1:1 conv, % diol:acetone >99 1 : 2 1 : 1 50
KINETIC RESOLUTION OF rac -1,4-BUTANDIOL Ar Ar Cp*RuCl(cod) Ar Ar chiral ligand OH OH OH KO t -Bu O + + OH OH OH acetone toluene O Ar Ar Ar Ar 30 °C, 1 h Ar = C 6 H 5 50:50 diol:acetone:Ru:chiral ligand:KO t -Bu = 100:100:1:1:1 ee, % a (reactant) R 1 , R 2 H, i -Pr 70 chiral ligand H, i -Bu 63 R 1 HN H, t -Bu 54 P(C 6 H 5 ) 2 H, Bn 79 R 2 H, Ph 50 -(CH 2 ) 3 - 23
SUMMARY Enantioselective lactonization of meso -1,4-butandiols O chiral ligand Cp*RuCl(cod) R R chiral ligand * OH KO t -C 4 H 9 H 2 N P(C 6 H 5 ) 2 O OH acetone * R R 30 °C, 1 h Bn >99% yield up to 50% ee Kinetic resolution of racemic 1,4-butandiol Ar Ar Cp*RuCl(cod) Ar Ar chiral ligand OH OH OH KO t -Bu O + + OH OH acetone OH toluene O Ar Ar Ar Ar 30 °C, 1 h 50:50 Ar = C 6 H 5 up to 79% ee
Recommend
More recommend