
CRITICALITY EXCURSION ANALYSIS TRACY Benchmark I IDENTIFICATION NUMBER: TRACY-LEU-SOL-STEP-001, 002, 003, 004, 005 KEY WORDS: Low enrichment, Solution, Step reactivity insertion 1.0 DETAILED DESCRIPTION Many experiments 1-7) have been performed to study criticality accident with solution fuel, however, obtained data have been not available in useful form for long time. TRACY Benchmark problem was made to provide experimental condition and obtained data of fission yield, power, temperature and pressure in organized form, which should be useful for the calculation by various criticality accident evaluation codes. The benchmark is expected to be beneficial to evaluate and/or improve such numerical codes through its analysis and to give the basis of the common knowledge of criticality accident. The feature of TRACY benchmark problem is as follows; - Low enriched uranyl nitrate solution, - Co-axial double cylinders tank, - Three methods of reactivity insertion. In this benchmark, the experiments of pulse reactivity insertion are provided, that is the simplest method of reactivity insertion for TRACY experiments. The selected experiment and its ID number are tabulated in Table 1.1. Table 1.1 Benchmark case and its excess reactivity. ID number Run number Excess reactivity ($) 001 100 0.30 002 143 0.70 003 72 1.10 004 196 2.00 005 203 2.97 1.1 Overview of Experiment Low enriched Uranyl nitrate aqueous solution is contained in a cylindrical core tank made of SUS304L stainless steel. There is a guide tube in its vertical center line for a transient rod, Tr-rod, which contains B 4 C inside it. After pumping fuel solution into the core tank, the transient rod is withdrawn from the core in order to insert reactivity. Desired reactivity is achieved by tuning the height of fuel solution. During free excursion, power, temperature and core pressure are measured. At the end, Tr-rod was inserted to shutdown. 1.2 Description of Experimental Configuration The core tank has an annular shape with 52cm outer and 7.6cm inner diameter. The effective cross section area for solution fuel is 1918 cm 2 . For more information, see Fig.1.1, 1.2 and 1.3. In Fig.1.3, the position 0.0mm of the Tr-rod means that the bottom of B 4 C inside the Tr-rod is 90mm below of the bottom of the fuel solution. The solution height is measured a needle type level gauge with accuracy of ± 0.25mm.
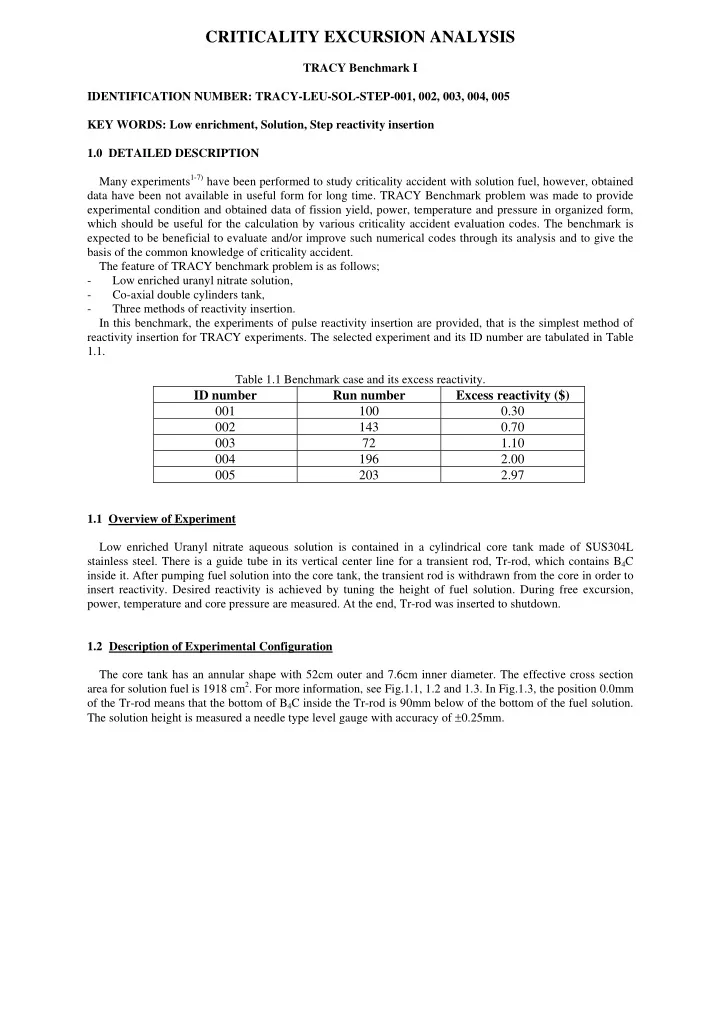
25 150 1875 3.5t Core bottom 80 250 10t 38.15 550 700 20 405 520 Figure 1.1: Schematic view of TRACY core tank.
Polyethylene moderator for Fission chamber OD47.0/ID7.0 mm Length = 100mm At 250mm from core bottom Thermocouples (Type-1) (to the center) Guide tube : SUS304L OD25.2/ID19.4 mm 195 140 100 150 80 500 75 130 Microwave level gauge Guide tube : Zircaloy OD22.94/ t 2.34 Pressure gauge 10 76.3 Core tank 3.5 50 42.7 36 15 150 5 28 50 Pressure gauge Core thermometer 80 Fuel feed/drain line SUS304L Core tank 100 OD34.0/ID28.0 support 30 ° Neutron source guide tube SUS304TP OD89.1/ t 4.0 10 (Unit : mm) Figure 1.2: Schematic view of TRACY core tank cross section (detail).
SUS304 (Cladding for Tr-rod) SUS304L (Core tank) B 4 C Hc (Critical solution level) 0.0 (Core bottom) -80.0 -90.0 -100.0 18.00 250.0 260.0 0.0 [13.40] 38.15 16.00 34.65 [9.90] 30.95 27.45 15.50 Unit : mm [9.40] The numbers in the bracket [ ] show the size for Rod I. Figure 1.3: Schematic view of cross section of TRACY core tank and Tr-rod.
1.3 Description of Material Data The fuel solution is uranyl nitrate solution, which consists of uranyl nitrate [UO 2 (NO 3 ) 2 ], free nitric acid [HNO 3 ], and water [H 2 O]. The enrichment of 235 U is 9.98 wt.%. Tables 1.2 through 1.6 give standard value of composition and atomic number density of materials. Table 1.2: Solution fuel conditions at 25 ° C. ID Run NO. I.R.($) U Conc.(gU/L) Acidity(N) 001 100 0.30 392.9 0.66 002 143 0.70 375.9 0.64 003 72 1.10 393.5 0.74 004 196 2.00 385.5 0.58 005 203 2.97 388.2 0.58 Table 1.3: Fuel conditions and kinetic parameters at 25.5 ° C. U-235 enrichment (%) 9.98 Uranium concentration (gU/L) 390 Acid morality (mol/L) 0.77 Solution height (cm) 50.88 Neutron multiplication factor[k eff ] 1.0111 7.5x10 -3 Effective delayed neutron fraction[ β eff ] Prompt neutron life time [ Λ ](sec) 4.6x10 -5 Table 1.4: Atom number densities of the fuel solution with the concentration of 390 gU/L at 25.5 ° C. Nucleus Number density (atoms/barn.cm) 5.7292x10 -2 H 2.4394x10 -3 N 3.7708x10 -2 O 9.9622x10 -5 U235 8.8823x10 -4 U238 Table 1.5: Atom number densities of SUS304L at 25.5 ° C. Nucleus Number density (atoms/barn.cm) 1.1939x10 -4 C 1.7004x10 -3 Si 1.7450x10 -2 Cr 1.7385x10 -3 Mn 5.7180x10 -2 Fe 8.9482x10 -3 Ni 4.4682x10 -5 Si Table 1.6: Atom number densities of air at 25.5 ° C. Nucleus Number density (atoms/barn.cm) 1.8789x10 -6 O16 5.7238x10 -6 N14
1.4 Description of External Neutron Source No external neutron source was used for each experiment. 1.5 Description of Initial States In each experiment, the reactivity is inserted by pulse withdrawal of the transient rod, and there was no external neutron source. Initial conditions are tabulated in Table 1.7. Table 1.7: Selected experiments and their initial states. Initial state Inserted Tr-rod ID Run NO. Criticality Sol. Fuel Temp. Reactivity($) position (Power) Level(mm) (°C) (mm) 001 100 0.30 Cri. (1W) 508.52 26.2 471.7 002 143 0.70 Sub 551.83 24.8 0.0 003 72 1.10 Sub 537.05 26.2 0.0 004 196 2.00 Sub 582.50 25.9 0.0 005 203 2.97 Cri. (1W) 623.76 26.1 0.0 1.6 Description of Reactivity Insertion In each experiment, a transient rod (Tr-rod) was fully inserted initially and was fully withdrawn within 0.2 seconds. Desired reactivity was achieved by following procedure; 1) The criticality solution height for which Tr-rod was fully withdrawn, H c1 , is measured. 2) The solution level, h , corresponding to desired reactivity for the experiment was determined by solving following formula; ⎧ ⎫ C 1 1 ρ = − − ⎨ ⎬ ( ) ( ) ⎭ I + λ 2 + λ 2 2 ⎩ h H c 1 where ρ I is desired reactivity, H cl : critical level of solution, C: constant; , and λ: = × 8 2 7 . 67 10 ( / ) C cent mm λ = extrapolation length . 102 mm ( ) 3) Solution level was tuned to the level h with Tr-rod fully inserted. 4) Tr-rod was withdrawn pneumatically at the time 0. 1.7 Description of the Detector or Measurement Systems and Measured Results Reactor power, fuel solution temperature and core pressure were measured. 1.7.1 Measurement of Power Levels – There were three neutron detectors. Two were linear channels and one was log channel. They were on ceiling of the core room right above the core tank as shown in Fig. 4. As the log detector, a cadmium covered 235 U fission chamber was employed, which was covered with 10mm-thick polyethylene and 1mm-thick cadmium in order to detect epi-thermal neutrons. It was placed in a lead shielding of 10cm-thick to reduce the noise due to gamma rays, which was about 2.5m far from the core. 1.7.2 Measurement of Temperature Levels and Distributions – Almel-chromel thermocouples were used to measure the temperature distribution in fuel solution. There were two types of thermocouple groups, they have different configuration of thermocouples to each other. Type-1 group was used for all experiments. Type-2 was used for R196 and R203 experiments. Type-1 has response time of about 1s and type-2, 0.1s.
1.7.3 Measurement of Pressure Levels – Core pressure was measured with a pressure gauge installed to the side wall of the core tank. The pressure was observed for more than 1.5$ of reactivity insertion. 2.0 EVALUATION OF DATA 2.1 Evaluation of Power Levels and Distributions There is a difference in measured values between linear channel and log channel. Such difference is less than 5% for reactivity lower than 1.5$. However, it increases as inserted reactivity increases. The difference is about 17% for R196(2.0$), about 48% for R203(2.97$). 2.2 Evaluation of Temperature Levels and Distributions Thermocouples have response of 0.1 to 1 second. The accuracy is ± 1.5 ° C. 2.3 Evaluation of Pressure Levels and Distributions The base line of the pressure decreases after the first peak power. That may be due to temperature change of solution fuel and the noises due to radiation. Amount of uncertainty is unknown.
Recommend
More recommend