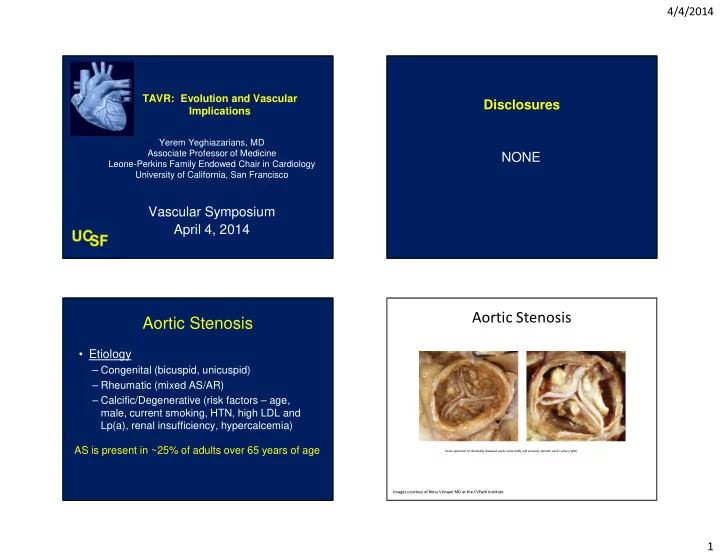

4/4/2014 TAVR: Evolution and Vascular Disclosures Implications Yerem Yeghiazarians, MD Associate Professor of Medicine NONE Leone-Perkins Family Endowed Chair in Cardiology University of California, San Francisco Vascular Symposium April 4, 2014 Aortic Stenosis Aortic Stenosis • Etiology – Congenital (bicuspid, unicuspid) – Rheumatic (mixed AS/AR) – Calcific/Degenerative (risk factors – age, male, current smoking, HTN, high LDL and Lp(a), renal insufficiency, hypercalcemia) AS is present in ~25% of adults over 65 years of age Gross specimen of minimally diseased aortic valve (left) and severely stenotic aortic valve (right) Images courtesy of Renu Virmani MD at the CVPath Institute 4 1
4/4/2014 Aortic Stenosis Aortic Stenosis • Classification of Severity – Mild (area 1.5 cm 2 , mean gradient <25 mmHg, jet velocity <3.0 m/sec) – Moderate (area 1.0-1.5 cm 2 , mean gradient 25-40 mmHg, jet velocity 3.0-4.0 m/sec) – Severe (area <1.0 cm 2 , mean gradient >40 mmHg, jet velocity >4.0 m/sec) NOTE: Do not rely on single number; Account for BSA; Symptoms predominate ~1/3 of patients with severe symptomatic aortic Poor Prognosis stenosis do not undergo AVR 35 8 5-Year Survival 30 30 28 25 23 Survival, % 20 15 12 10 5 4 3 0 Breast Lung Colorectal Prostate Ovarian Severe Cancer Cancer Cancer Cancer Cancer Inoperable AS* *Using constant hazard ratio. Data on file, Edwards Lifesciences LLC. Analysis courtesy of Murat Tuczu, MD, Cleveland Clinic 5 year survival of breast cancer, lung cancer, prostate cancer, ovarian cancer and severe inoperable aortic stenosis 1. Bouma B J et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 1999;82:143-148 2. Iung B et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European Heart Journal 2003;24:1231-1243 (*includes both Aortic Stenosis and Mitral Regurgitation patients) 8 3. Pellikka, Sarano et al. Outcome of 622 Adults with Asymptomatic, Hemodynamically Significant Aortic Stenosis During Prolonged Follow-Up. Circulation 2005 4. Charlson E et al. Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis2006;15:312-321 2
4/4/2014 TAVR Edwards SAPIEN Transcatheter Heart Valve Transfemoral and Transapical Leaflets matched for thickness Bovine pericardial tissue and elasticity Stainless steel frame PET skirt Transapical Transfemoral 10 Study Devices Ensuring the Appropriate Annular Size Range • The Edwards SAPIEN transcatheter heart valve is offered in two sizes, 23 mm and 26 mm, and accommodates an annular size range of 18 mm to 25 mm Valve Size to Annulus Diameter Annulus diameter 18 mm 19 mm 20 mm 21 mm 22 mm 23 mm 24 mm 25 mm by TEE 23 mm or 26 mm Edwards SAPIEN THV RetroFlex Ascendra valve 23 and 26 mm valves 22 and 24 F sheaths 24 and 26 F sheaths 12 3
4/4/2014 Inoperable PARTNER Cohort PARTNER Study Design Primary Endpoint: All-Cause Mortality HR [95% CI] = Symptomatic Severe Aortic Stenosis Standard Rx 0.54 [0.38, 0.78] TAVI ASSESSMENT: High-Risk AVR Candidate ASSESSMENT: High-Risk AVR Candidate P (log rank) < 0.0001 3,105 Total Patients Screened 3,105 Total Patients Screened All-cause mortality (%) Total = 1,057 patients N = 358 N = 699 Inoperable Inoperable High Risk High Risk 2 Parallel Trials: ∆ at 1 yr = 20.0% 50.7% Individually Powered NNT = 5.0 pts ASSESSMENT: ASSESSMENT: Transfemoral Transfemoral Access Access Yes No 30.7% 1:1 Randomization Not In Study N = 179 N = 179 Standard TF TAVR Therapy VS Leon et al, NEJM 2010; 363:1597-1607 Months Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) Numbers at Risk Co-Primary Endpoint: Composite of All-Cause Mortality and Repeat Hospitalization (Superiority) TAVI 179 138 122 67 26 Standard Rx 179 121 83 41 12 Primary Endpoint: PARTNER Study Design All-Cause Mortality at 1 Year Symptomatic Severe Aortic Stenosis HR [95% CI] = 0.5 TAVR 0.93 [0.71, 1.22] ASSESSMENT: High-Risk AVR Candidate ASSESSMENT: High-Risk AVR Candidate 3,105 Total Patients Screened 3,105 Total Patients Screened AVR P (log rank) = 0.62 0.4 Total = 1,057 patients N = 699 Inoperable Inoperable N = 358 High Risk High Risk 2 Parallel Trials: 26.8 0.3 Individually Powered ASSESSMENT: ASSESSMENT: ASSESSMENT: ASSESSMENT: Yes No Transfemoral Transfemoral Transfemoral Transfemoral 0.2 24.2 Access Access Access Access Transfemoral (TF) Transapical (TA) Yes No 0.1 1:1 Randomization 1:1 Randomization 1:1 Randomization Not In Study 0 N = 244 N = 248 N = 104 N = 103 N = 179 N = 179 0 6 12 18 24 Standard TF TAVR AVR TA TAVR AVR TF TAVR Therapy No. at Risk Months VS VS VS Primary Endpoint: All-Cause Mortality Primary Endpoint: All-Cause Mortality at 1 yr TAVR 348 298 260 147 67 Over Length of Trial (Superiority) (Non-inferiority) Co-Primary Endpoint: Composite of All-Cause Mortality AVR 351 252 236 139 65 and Repeat Hospitalization (Superiority) 4
4/4/2014 Neurological Events at 30 Days and 1 Year All Patients (N=699) Major Vascular Complications 30 Days 1 Year AT Population TAVR AVR TAVR AVR p-value p-value Outcome (N = 348) (N = 351) (N = 348) (N = 351) All Stroke or TIA – no. (%) 19 (5.5) 8 (2.4) 0.04 27 (8.3) 13 (4.3) 0.04 TIA – no. (%) 3 (0.9) 1 (0.3) 0.33 7 (2.3) 4 (1.5) 0.47 All Stroke – no. (%) 16 (4.6) 8 (2.4) 0.12 20 (6.0) 10 (3.2) 0.08 Major Stroke – no. (%) 13 (3.8) 7 (2.1) 0.20 17 (5.1) 8 (2.4) 0.07 Minor Stroke – no. (%) 3 (0.9) 1 (0.3) 0.34 3 (0.9) 2 (0.7) 0.84 Death/maj stroke – no. (%) 24 (6.9) 28 (8.2) 0.52 92 (26.5) 93 (28.0) 0.68 18 THE PARTNER TRIAL COHORT A Courtesy of Sandeep Nathan, MD Major Bleeding TAVR Vascular Complications AT Population JACC 2012; 1043-1052 19 THE PARTNER TRIAL COHORT A Courtesy of Sandeep Nathan, MD 5
4/4/2014 TAVR Vascular Complications Assessing Appropriate Vascular Access Vessel diameters must be a minimum of: – ≥ 7 mm for a 23 mm valve (requires a 22F RetroFlex 3 sheath) – ≥ 8 mm for a 26 mm valve (requires a 24F RetroFlex 3 sheath) JACC 2012; 1043-1052 22 Courtesy of Sandeep Nathan, MD TAVR: Present & future TAVR: Present & future Medtronic CoreValve TM Medtronic CoreValve TM Edwards Sapien XT (2 nd Medtronic Engager St. Jude Medical generation) 18/19 Fr with TA valve (Ventor Portico valve NovoFlex delivery system Embracer) • 18 Fr delivery system Edwards Sapien THV (1 st • Transfemoral or subclavian delivery generation) 22/24 Fr with Boston Scientific JenaValve • Repositionable, self-expanding system RetroFlex 3 delivery system Lotus self-exp valve (Sadra Lotus) • Perhaps greater need for pacing afterwards Medtronic CoreValve 6
4/4/2014 91 year old with severe AS requiring a 26 mm Edwards valve (TAVR) Requires a 24F RetroFlex 3 sheath Up and around angio of the left side and advancement of pigtail to the left common femoral artery for optimal TAVR access Right groin access � Preclose with 3 Perclose Devices Successful TAVR with 26 mm valve under rapid pacing Advanced a 300 cm Steel Core wire to the left SFA of 180 beats/min to induce cardiac standstill via a crossover sheath 7
4/4/2014 Future of TAVR – it’s very bright! Advanced a 10mm x 4cm Charger balloon to the left external iliac over the Steel Core wire and Perclosed the 3 Preclose devices. Then Inflated the balloon to 1 atm for 5 mins at the femoral head 1. Lower profile devices 2. Repositionable devices 3. Retrievable devices 4. Paravalvular leak 5. Delivery system options 6. Eliminate need for pacing 7. Embolic protection Courtesy of Sandeep Nathan, MD Impact of Total AR on Mortality (AT) Question 1: Is TAVR FDA approved for use TAVR Patients in Bicuspid Aortic Valve Stenosis? 60.8% 53.7% 1. Yes 44.6% 67% 2. No 38.2% 32.5% 35.3% 26.0% 25.6% 33% 12.3% No. at Risk None-Tr 131 121 114 102 93 80 63 Mild 171 146 125 117 110 94 62 Yes No Mod-Sev 34 24 21 18 15 12 9 8
4/4/2014 Question 2: Is TAVR FDA approved for use as treatment for Aortic Valve Regurgitation? Thank You 1. Yes 79% 2. No 21% s o e N Y 9
Recommend
More recommend