
1
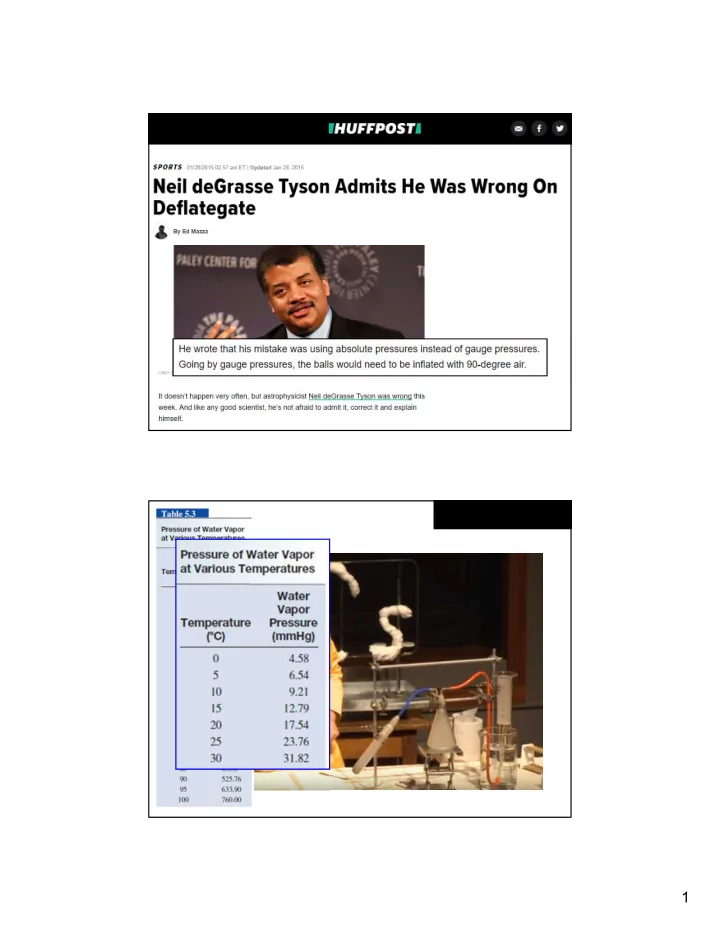
The pressure on a diver increases by 100 kPa (1.00 atm) for every 10 m the diver descends. For dives deeper than 66 m the gas mixture should contain less than 21% oxygen to avoid the risk of acute oxygen toxicity. The general rule is to try to achieve a gas mixture giving an Fio2 of about 140 kPa. At 130 m depth in the northern sector of the North Sea oil field, the ambient pressure is 1400 kPa, so the breathing mixture used contains 10% oxygen. On the deepest working dives, at depths greater than 600 m, ambient pressure is greater than 6100 kPa and the divers breathe gas mixtures containing about 2% oxygen to avoid acute oxygen toxicity. A lung full of gas containing 2% oxygen at 600 m contains about six times as many molecules of oxygen as a lung full of air at sea level. On deep dives the composition of the gas breathed is changed several times during descent and ascent. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1114047/ 600 m => 6100 kP => 60 atm! 60 x (2%/100%) => P(O2) = 1.2 atm https://en.wikipedia.org/wiki/Partial_pressure#In_medicine 2
http://slideplayer.com/slide/4772848 / . P V = nR T P P P nR nR 1 2 as s uming V is cons tant => = = > = = T V T V T 1 2 P P P P 1 2 i f S o = or = T i = 23 o C + 273.15 = 296 K T T T T 1 2 i f T f = 9 o C + 273.15 = 282 K Rearranging this expression, the final pressure at halftime would be P T 27.2 ps i x 282 K i f P = = = 25.9 ps i f T 296 K i 25.9 psi (absolute) - 14.7 = 11.2 psi-g Below the 12.5 - 13.5 “psi” required by the NFL, but not 2 psi below the minimum allowed pressure 3
2NaN 3 ( s ) 2Na( s ) + 3N 2 ( g ) https://www.youtube.com/watch?v=IzC_QqKhTQg 4
5
http://www.ncert.nic.in/html/learning_basket/energy10class/combustion%20engine1.htm 6
fuel + O 2 CO 2 + H 2 O chemical energy (fuel) you add energy you release (light, heat, motion) food + O 2 CO 2 + H 2 O chemical energy (food) you add energy you release (heat, to maintain body temperature; motion, to do work) 7
8
9
We use stored energy from food at a rate ranging from about 1 Cal/min (basal metabolic rate) to about 15 Cal/min. 10
Assumptions: No exercise beyond the normal activity to burn 2000 Calories per day. No food. Get energy from glycogen until gone, then burn fat after that. http://www.uic.edu/classes/phar/phar332/Clinical_Cases/carbo%20metab%20cases/ glycogen%20metab/Glycogen%20biochemistry.htm 11
Assuming an energy need of 2000 Cal/day and no food (!) during this time: 2000 Cal/day (1 g/9 Cal) (1 lb/453.6 g) 0.4899 lb/day So, max weight loss in 20 days is 1 lb (1 st day, glycogen) + 19 day(0.5 lb/day) = 10.5 lb ! Assuming an energy need of But what if protein is 2000 Cal/day and no food (!) “burned” for energy instead during this time: of fat 2000 Cal/day 2000 Cal/day (1 g/9 Cal) (1 g/4 Cal) (1 lb/453.6 g) (1 lb/453.6 g) 0.4899 lb/day 1.1 lb/day So, max weight loss in 20 So, max weight loss in 20 days is days is 1 lb (1 st day, glycogen) 1 lb (1 st day, glycogen) + 19 day(0.5 lb/day) + 19 day(1.1 lb/day) = 1 lb + 19(0.5) = 10.5 lbs ! = 1 lb + 19(1.1) = 22 lbs !? 12
Figure 5.19 The internal energy, U, of a system can be changed by heat flow and work. If heat flows into the system, q in (q > 0), or work is done on the system, w on (w > 0), its internal energy increases, ΔU > 0. If heat flows out of the system, q out (q < 0), or work is done by the system, w by , (w < 0), its internal energy decreases, ΔU < 0. 13
2:1 H 2 O 2 H 2 +O 2 Which balloon contains the most molecules? A. the H 2 balloon B. the O 2 balloon C. the H 2 /O 2 balloon D. they all contain the same number of molecules 14
2:1 H 2 O 2 H 2 +O 2 Which balloon is the heaviest? A. the H 2 balloon B. the O 2 balloon C. the H 2 /O 2 balloon D. all three balloons are equally heavy 2:1 H 2 O 2 H 2 +O 2 Which balloon is the densest? A. the H 2 balloon B. the O 2 balloon C. the H 2 /O 2 balloon D. all three balloons are equally dense 15
2:1 H 2 O 2 H 2 +O 2 Which balloon released the most energy? A. the H 2 balloon B. the O 2 balloon C. the H 2 /O 2 balloon D. all three balloons are equally dense 16
Recommend
More recommend