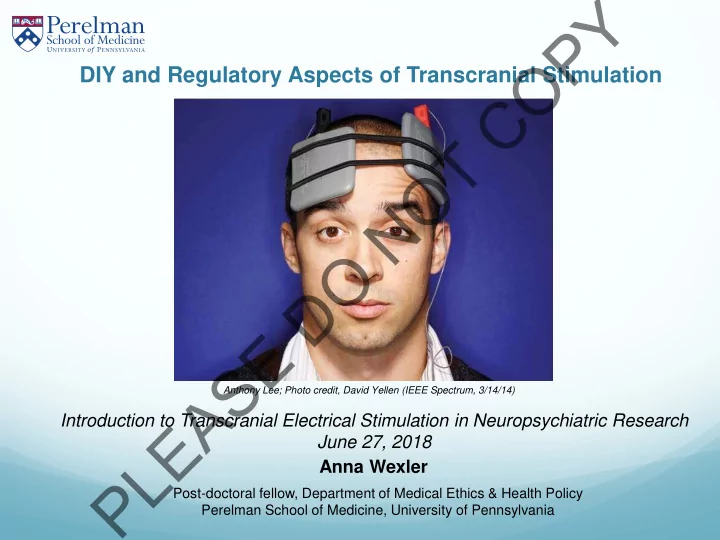

Y P O DIY and Regulatory Aspects of Transcranial Stimulation C T O N O D E S A Anthony Lee; Photo credit, David Yellen (IEEE Spectrum, 3/14/14) Introduction to Transcranial Electrical Stimulation in Neuropsychiatric Research E June 27, 2018 L Anna Wexler P Post-doctoral fellow, Department of Medical Ethics & Health Policy Perelman School of Medicine, University of Pennsylvania
Y P Talk Outline O C Do-it-yourself and direct-to-consumer tDCS T O Who are home users, what devices do they use, how and why do they stimulate, and do they find tDCS effective? N O Regulation of tCS devices in the US D FDA medical device law & tDCS devices E S A E L P
Y P Number of academic journal publications about tDCS by year (2000-2016) O 500 C 450 T 400 O 350 N 300 O 250 D 200 150 E S 100 A 50 E 0 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 L P
Y P O C T O N O D E S A E L P Dubljevic V, Saigle V, & Racine E. (2014). “The Rising Tide of tDCS in the Media and Academic Literature ,” Neuron ( 82)731-736, DOI:10.1016/j.neuron.2014.05.003
Y P Rise of DIY tDCS O C T DIY tDCS O N O D E S A E L P
Y P Do-it-yourself device O C T O N O D E S A E L P
Y P Repurpose an Iontophoresis Device O C T O N O D Chattanooga Ionto™ E S A E L P ActivaDose II
Y P Current source/tDCS device O C T O N O D E S Super Specific Devices A E Caputron Medical L P
Y P tDCS Devices and “Device Kits” O C T O N O tDCS-Kit D PriorMind Brain Stimulator E S A E L P Cognitive Kit Apex Type A
Y P Other tDCS Devices O C T O N O D E S A E L P TCT Research Limited (Hong Kong)
Y P Wearable Devices O C T O N O D E S A E L P
Y P Blurred boundaries between do-it-yourself and direct-to- O consumer C T O N O D Do-it-yourself (DIY) Direct-to-consumer (DTC) E S A E L P
Y P Studying home users of tDCS O C Ethnography of home users (Wexler 2015) T • Analysis of DIY tDCS websites, forums and blogs O • Semi-structured interviews with home users N O Survey of consumers of tDCS devices (Wexler 2018) D • 7 companies agreed to participate on the condition of anonymity; emails sent to companies’ customer lists with link to online survey E • Quantitative and open-ended qualitative questions about tDCS S device(s), use practices, beliefs, attitudes, and demographics A E L P
Y P Who are home users? O 300 250 339 respondents C 200 n = 339 327 (96.5%) for self-use 150 T and 12 (3.5%) on others 100 O 50 0 N Central Country of residence Male (83.5%) Female (15.3%) Prefer not to and (n=339) answer (1.2%) South America O (2.4%) Asia (2.7%) 60 D 50 E Europe 40 (15.9%) n = 339 Australia & S New Zealand 30 (5.3%) A North America (73.5%) 20 E 10 L P 0 Participants' Ages (in years; mean 45.3) (Wexler 2018)
Y P Who are home users? O C • Wealthy T • Highly educated (77.9% have a college degree or higher; 36.5% Master’s or higher) O N • Politically liberal (70.5%) O • Early adopters of technology (63.7%) D • Read articles about science frequently or very frequently (82.3%) E • Never or rarely attend religious services (77.9%) S • Nearly half have used dietary supplements or non-prescription drugs to A improve cognition E L P (Wexler 2018)
Y P B. Treatment, enhancement and restoration: user combinations O A. Treatment, enhancement and (n=308) restoration: total numbers None of the 250 C three (3.2%) Only restoration (2.3%) Treatment & restoration (4.2%) T 200 O Treatment, restoration & N enhancement 150 (9.1%) O n=308 Only enhancement Restoration & (41.2%) D Enhancement (10.7%) 100 E Only treatment (13.3%) S 50 A E Treatment & 0 enhancement (15.9%) Enhancement Treatment Restoration L (76.9%) (42.5%) (26.3%) P (Wexler 2018)
Y P O C T O N O D E S A E L P (Wexler 2018)
Y Treaters vs. Non-treaters, by gender Treaters vs. Non-treaters, by ratings of success P of tDCS 100% 100% O 90% 90% C 80% 80% 73.3% 70% T 70% O 60% 65.4% 61.8% 60% 56.2% N 50% 50% 40% 43.8% 40% O 38.2% 34.6% 30% 30% D 26.7% 20% 20% E 10% 10% S 0% 0% Found tDCS unsuccessful (n=81) Found tDCS successful (n=130) Male (n=251) Female (n=45) A Treater Non-treater Treater Non-treater E [ X 2 (1, N =296)=19.11, p <.001; Cramer’s V = .254] [ X 2 (1, N =211)=9.32, p =.002; Cramer’s V = .210] L P (Wexler 2018)
Y P How do home users learn about stimulation parameters? O C T • link to scientific articles (when behind firewall, post unrestricted copies) O • use video tutorials on electrode positioning N O D E S A E L P (Wexler 2015)
Y P How do home users learn about stimulation parameters? O C T O N Home users transform existing O scientific literature into user-friendly D indexes and guides geared E towards their needs S A E L P (Wexler 2015)
Y P How many times have you administered tDCS to yourself? O 80 C 70 T 60 O 50 N n=308 40 O 30 D 20 E 10 S A 0 5 or less 6 to 10 11 to 20 21 to 49 50 to 99 100+ (8.4%) E (20.5%) (14.3%) (23.4%) (19.5%) (13.6%) L Home users mostly adhere to established scientific protocols (e.g., current level & session duration) but P depart regarding number of stimulation sessions (Wexler 2018)
Y P To what extent did you feel that your use of tDCS was successful? O 120 C 100 T O 80 N n=308 60 O D 40 E 20 S A 0 E Totally unsuccessful Somewhat Not Sure (29.9%) Somewhat successful Totally successful (16.2%) unsuccessful (11.4%) (32.1%) (10.4%) L P (Wexler 2018)
Y P Did you experience unwanted side effects from tDCS? If yes, please describe. O 140 C 120 T 100 O N 80 n=308 O 60 D 40 E S 20 A 0 E No (38.0%) Skin irritation "Skin burn" or Headache Flash of light Dizzinesss Metallic Taste (35.4%) "burning" (10.1%) (phosphene) (1.9%) (1.5%) L sensation (8.4%) (16.9%) P 10 reports of serious skin burn (Wexler 2018)
Y P Current Users, Formers Users, and Never Used O C Never used (5.8%; n=19) T O Frequently: 3+ sessions per week (20.2%; n=66) N Several times a month, (14.1%; n=46) Current user (59.3%; O Former user (34.9%; n=194) n=114) D In fits and spurts (25.1%; n=82) E S A (n=327) E L P (Wexler 2018)
Y P Practices of DIY Brain Stimulation O C T Recognize that home users are utilizing tDCS both for treatment and O enhancement N Be aware that an unintended “second audience” is utilizing published scientific research O Be prepared for individuals to approach you for guidance D Informing home users/DIYers of risks (Wurzman et al. 2016) E S A E L P
Y P Are consumer non-invasive brain stimulation devices O considered medical devices under US law? C T O N O D Do-it-yourself (DIY) Direct-to-consumer (DTC) E S A E L P
Y P O C T O N O D E S A E L P
Y P O C T Food & Drugs Act (1906) O N - Prohibited misbranded & adulterated food and drugs O Federal Food Drug & Cosmetic Act (1938) D - Granted FDA limited jurisdiction over medical devices E Medical Device Amendments (1976) S A - Device manufacturers required to notify FDA of medical E device prior to marketing L P
Y P Risk-based classification framework O Class I Class II Class III C Low risk Moderate risk High risk T O N O D E Most products exempt Pre-market notification Pre-market S from pre-market (PMN) required via approval (PMA)— notification 510(k). Devices are must demonstrate A “cleared.” safety & efficacy E L P
Y P FDA approved/cleared stimulation devices O Class I Class II Class III C Low risk Moderate risk High risk T TENS (pain/headache) O DBS (Parkinson’s related) N rTMS (treatment-resistant MDD) ECT (severe O depression)* TMS (headache) D VNS (epilepsy- tVNS (cluster headache) related) E S CES (depression, insomnia* & A anxiety*) E L P *Subject of recent proposed order to reclassify
Y P O Definition of a Medical Device C According to Section 201(h) of the Food, Drug & Cosmetic (FD&C) Act, a medical T device is: O an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or N accessory which is: O recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, D intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, E or S intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through A chemical action within or on the body of man or other animals and which is not E dependent upon being metabolized for the achievement of any of its primary intended purposes. L P
Recommend
More recommend