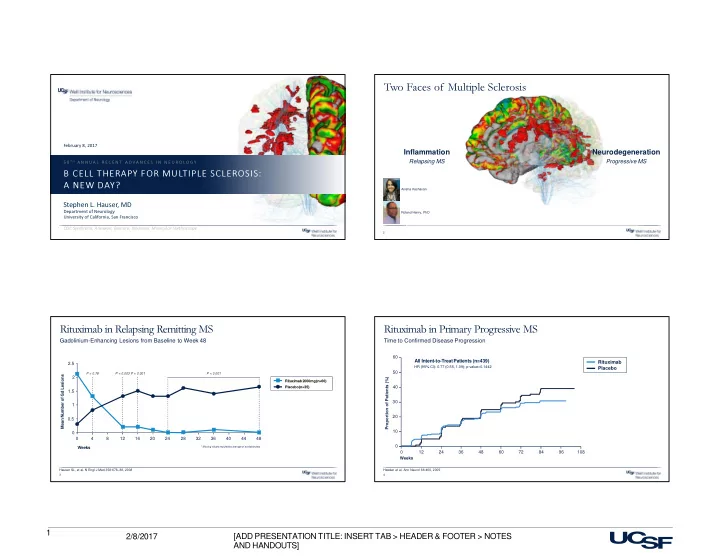

Two Faces of Multiple Sclerosis February 8, 2017 Inflammation Neurodegeneration Relapsing MS Progressive MS 5 0 T H A N N U A L R E C E N T A D VA N C E S I N N E U R O L O G Y B CELL THERAPY FOR MULTIPLE SCLEROSIS: A NEW DAY? Anisha Keshavan Stephen L. Hauser, MD Department of Neurology Roland Henry, PhD University of California, San Francisco COI: Symbiotix, Annexon, Bionure, Neurona, Molecular Stethoscope 2 Rituximab in Relapsing Remitting MS Rituximab in Primary Progressive MS Gadolinium-Enhancing Lesions from Baseline to Week 48 Time to Confirmed Disease Progression 60 All Intent-to-Treat Patients (n=439) Rituximab 2.5 HR (95% Cl): 0.77 (0.55, 1.09); p-value=0.1442 Placebo 50 P = 0.78 P = 0.003 P = 0.001 P < 0.001 Mean Number of Gd Lesions 2 Proportion of Patients (%) Rituximab 2000mg (n=66) 40 Placebo (n=35) 1.5 30 1 20 0.5 10 0 0 4 8 12 16 20 24 28 32 36 40 44 48 0 Weeks * Missing values imputed by average of available data 0 12 24 36 48 60 72 84 96 108 Weeks Hauser SL, et al. N Engl J Med 358:676–88, 2008 Hawker et al, Ann Neurol 66:460, 2009 3 4 1 2/8/2017 [ADD PRESENTATION TITLE: INSERT TAB > HEADER & FOOTER > NOTES AND HANDOUTS]
Ocrelizumab in Relapsing Remitting MS: OPERA I & II: Two Identical Studies of Ocrelizumab in Relapsing MS Reduction in Gd-Enhancing Lesions Maintained Through 144 Weeks Patients with baseline MRI 4.0 1:1 Randomization Mean No. T1 Gd-enhancing Lesions OLE screening period Placebo (n=54) Ocrelizumab600mg (n=55) • RMS diagnosis 3.0 Ocrelizumab1000mg (n=55) • 18–55 yrs Primary end point: IFN beta-1a (n=54) OLE • EDSS of 0.0–5.5 OCR vs placebo • ≥2 clinical 2.0 - ‘Core Study' (0–96 weeks) relapses within - ‘Follow-Up' (97–144 weeks) a last 2 yrs or 1 relapse in last yr 1.0 Safety follow-up * * ≈48 weeks from date of last infusion 0 0 4 8 12 16 20 24 48 72 96 120 144 B-cell monitoring ‡ Weeks ‡Con�nued monitoring occurs if B cells are not repleted. *p<0.0001 for both OCR doses vs placebo, N (for primary analysis): Placebo=54, OCR 600 mg=51, OCR 1000 mg=52, IFN-β1a=52 a Patients who withdrew during earlier treatment cycles were also included in the follow-up periods EDSS, Expanded Disability Status Scale; IFN, interferon; i.v., intravenous; OLE, open-label extension; RMS, relapsing multiple sclerosis; s.c., subcutaneous. Hauser SL, et al. Presented at the American Academy of Neurology, March 13, 2013 5 6 MS Disease Histories and Characteristics Were Balanced Over 85% of Ocrelizumab Patients Completed the Studies OPERA I OPERA II OPERA I OPERA II IFN β -1a Ocrelizumab IFN β -1a Ocrelizumab 44 µ g 600 mg 44 µ g 600 mg IFN β -1a Ocrelizumab IFN β -1a Ocrelizumab n=411 n=410 n=418 n=417 44 µ g 600 mg 44 µ g 600 mg Age, yr, mean (SD) 36.9 (9.3) 37.1 (9.3) 37.4 (9.0) 37.2 (9.1) ITT*, n 411 410 418 417 Female, n (%) 272 (66.2) 270 (65.9) 280 (67.0) 271 (65.0) Treated, n 409 408 417 417 Time since onset, yr, mean (SD) 6.3 (6.0) 6.7 (6.4) 6.7 (6.1) 6.7 (6.1) Time since diagnosis, yr, mean (SD) 3.7 (4.6) 3.8 (4.8) 4.1 (5.1) 4.2 (5.0) Withdrawn, n (%) 69 (17) 42 (10) 97 (23) 57 (14) Relapses previous 12 months, mean (SD) 1.3 (0.6) 1.3 (0.7) 1.3 (0.7) 1.3 (0.7) Withdrawn due to AE, n (%) 25 (6.1) 13 (3.2) 25 (6.0) 16 (3.8) Entered safety follow-up, n (%) 42 (61) 24 (57) 39 (40) 16 (28) Previously untreated*, n (%) 292 (71.4) 301 (73.8) 314 (75.3) 304 (72.9) EDSS, mean (SD) 2.8 (1.3) 2.9 (1.2) 2.8 (1.4) 2.8 (1.3) Completed, n (%) 340 (83) 366 (89) 320 (77) 360 (86) Gd + lesions, n (%) 155 (38.1) 172 (42.5) 172 (41.4) 161 (39.0) Entered safety follow-up, n (%) 12 (4) 10 (3) 15 (5) 8 (2) Number Gd + T1 lesions, mean (SD) 1.9 (5.2) 1.7 (4.2) 2.0 (4.9) 1.8 (5.0) Entered open-label extension, n (%) 326 (96) 352 (96) 297 (93) 350 (97) Number T2 lesions, mean (SD) 51.1 (39.9) 51.0 (39.0) 51.0 (35.7) 49.3 (38.6) ITT *Untreated in 2 years prior to study entry. *All randomized patients will be included in the ITT population. Patients prematurely withdrawing from the study for any reason and for whom an assessment was not performed EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; IFN, interferon; SD, standard deviation; yr, year. for whatever reason will still be included in the ITT analysis. AE, adverse event; IFN, interferon; ITT, intent to treat. 7 8 2 2/8/2017 [ADD PRESENTATION TITLE: INSERT TAB > HEADER & FOOTER > NOTES AND HANDOUTS]
Ocrelizumab in Relapsing MS Ocrelizumab in Relapsing MS Reduction in Annualized Relapse Rate Compared With IFN β -1a Reduction in Mean Gadolinium-Enhancing Lesions Compared With IFN β -1a OPERA I OPERA II OPERA I OPERA II 46% 47% ARR reduction vs ARR reduction 97% IFN β -1a vs IFN β -1a p<0.0001 p<0.0001 p<0.0001 95% p<0.0001 96% 92% p<0.0001 p<0.0001 98% 91% p<0.0001 p<0.0001 ITT ITT *Adjusted ARR calculated by negative binomial regression and adjusted for baseline EDSS score (<4.0 vs ≥4.0), and geographic region (US vs ROW). *Adjusted by means calculated by negative binomial regression and adjusted for baseline T1 Gd lesion (present or not), baseline EDSS (<4.0 vs ≥4.0) and geographical region (US vs ROW). ARR, annualised relapse rate; EDSS, Expanded Disability Status Scale; IFN, interferon; ROW, rest of the world. EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; IFN, interferon; MRI, magnetic resonance imaging; ROW, rest of the world. 9 10 Reduction in Pre-specified Pooled Analysis of Higher Proportion of Patients With Improvement in Confirmed Disability Progression (CDP) Brain Volume Loss Compared With IFNβ-1a OPERA I OPERA II Time to CDP for ≥ 12 weeks Time to CDP for ≥ 24 weeks Percentage Change in Brain Volume from Percentage Change in Brain Volume from Baseline to Week 96 * Baseline to Week 96 † Week Week 15.2 12.0 9.8 7.6 Risk reduction: 40% Risk reduction: 40% HR (95% CI): 0.60 (0.45, 0.81); p=0.0006 HR (95% CI): 0.60 (0.43, 0.84); p=0.0025 ITT ITT Exploratory endpoints: *24% improvement vs IFN β-1a; p<0.0001; †24% improvement vs IFN β-1a; p=0.0001; CI, confidence interval; HR, hazard ratio; IFN, interferon; OCR, ocrelizumab. ‡Compared using the Cochran–Mantel–Haenszel test stratified by geographic region (US vs ROW) and baseline EDSS score (<4.0 vs. ≥4.0). 11 12 3 2/8/2017 [ADD PRESENTATION TITLE: INSERT TAB > HEADER & FOOTER > NOTES AND HANDOUTS]
Recommend
More recommend