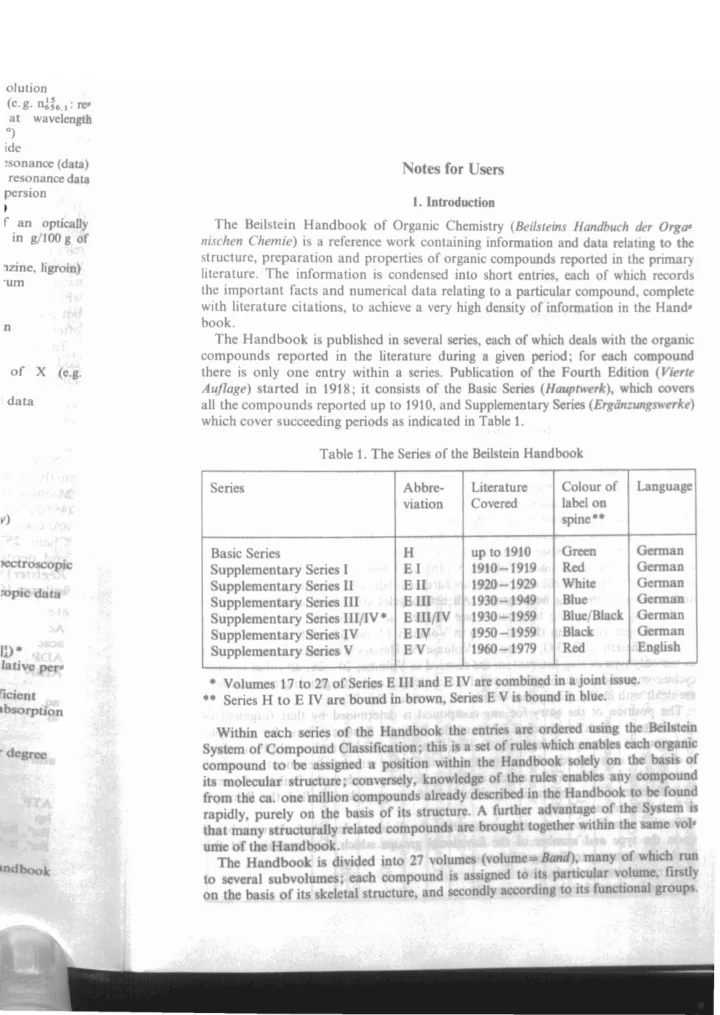

o/ution (e.g. n~~6 .I : I'e" at wavelength 0) ide esonance (data) Notes for Users resonance data persion I. Introduction I r an optically The Beil stein Handbook of Organic Chemistry (Beilsteins Handbuch der Orgo« in g/100 g of nischen Chem ie) is a reference work containing information and data r lating to th structure, preparation and properties of organic compounds reported in the prima ry 1Zine, ligroin) lit erature . The information is condensed into short entries, each of which record 'urn the important facts and numerical data relating to a particular compound , complete with literature citations, to achieve a very high density of information in the Hand - book . n The Handbook is published in several eries, each of which deal with th organic compounds reported in the literature during a given p riod: for each compound of X (e.g. there is only one entry within a series . Publication of th Fourth Edition (Vierte Auflage) started in 1918; it consists of the Basic Seri s (Hauptwerk), which cove data all the compounds reported up to 1910, and Supplementary ri (Ergdnzungswerke) which cover succeeding periods as indicated in Table 1. Table 1. The Serie of the Beilstein Handbook Colour of Langua Serie Abbre- Literatur Covered label on viation pine·· v) G n H up to 1910 Basic Series >ectro pi 1910-1919 Red I EI Supplementary Serie II Ell 1920-1929 Whit Supplementary S ri pi d ta 19 0-1 49 Blu EIII Suppl m nt ry Seri III Blu /BI c 1930-1959 III /IV· III/IV Suppl mentary S rie BI ck 1950-1959 IV IV Supplementary Serie lU- 1 60-1979 Red V EV Supplem ntary eri I tiv p • Volume 17 to 27 of Serie E III and E IV are combin d in a j int i 'i j nt •• Series H to E IV are bound in brown, Seri V i b und in blu . rb rption Within each erie of the Handbo k th entri Sy tern of ompound Cia ification; thi i a t of rul -d comp und to be a ign d po ition ithin th Handb i molecular tru tur ; conver ely, knowl d of th rul
XII Not es for Users Within th individual volumes the ordering is based on structural features such as th type and number of functional group s, the degree of unsaturation, the number of carbon tom and other similar criteria, until each compo und has been assigned po iti n within the eries. To u e the Handbook effectively, however. it is not i n ry to no all the rule of the Beilstein System, but merely to be able to id ntify in which of the 27 volumes the compound is described; this is outlined in tion . A more detailed description with worked examples is to be th following found in the b 0 1 t .. Ho to U e Beilstein" (also available in German) which. t th r wi h oth r inti rmativ mat rial about the Beilstein Handbook. may be ob- t in fr f h rg by writing to Springer-Verlag Beil tein-In titut Abt. 4005 fiir Lit r tur der Organi chen Chemic Varrentrappstra e 40 -42 Tiergartenstrasse 17 0-60 Frankfurt /M. 90 0-6900 Heidelberg I u r of the Beilstein Handbook who are unfamiliar with the German a p cket-size " Beil tein Dictionary" (German/Engli sh) has been compiled il t in ditorial staff and i al 0 available free of charge. The contents thi dicti nary rio to be found on the green pages in either of the subvolumes I /IV 22/7 or IV 6/4. 2. tin Compound in the Beilstein Handbook Th b t way of finding th ntry for a particular compound in the Beilstein Hand - book i to identify in which volum th compound is listed, and then to consult th appr priat volume inde , d tail of which are given in section 4. In order to identify th volum of int re t a f ba ic principles of the Beilstein System need to und r tood and the ar d ri d below. or th purp of cla ification in the B il t in System, all organic compounds are divid into thr ba ic type, viz. acyclic compounds (Volumes 1 -4) , isocyclic (i. . carbocyclic) compound (Volume 5 - 16), and heterocyclic compounds (Vol- 17 - 27). H t ro yclic compound are furth r subdivided according to the natur e urn and numb r of th ring heteroatom . All heterocycles containing chalcogen atom s a th only typ f ring heteroatom are covered in Volumes 17 - 19; those with on chalcogcn rin atom (het ro: 1 0) in Volumes 17 and 18, and those with two or mor (h tero: 20, 30, ... et .) in Volume 19. Heterocycles containing nitrogen a. th only typ of ring heteroatom ar cover din Volum 20 -26 ; all other hetero - cyclic comp und , including tho with b th chalcogen and nitrogen ring heteroatoms, ar d alt with in Vol. 27 (furth r detail r given in Table 2). The po ition of the entry for any compound is determined by that fragment of it tru tur which i cla iii d late t in th Beil t in Sy t m. Thus, for example, a comp und ntaining a h t rocycli ring, a carbocyclic ring and an aliphatic chain i cl ifi d a a h ter cycl , irre p tiv of th other tructural elements, since all h t rocycl rd r dafter acycli and i ycliccompounds in the Beilstein System. Thi f atur of th y tcmatic cla iii tion of compound i called the Principle of Lat t y t mati Entry. urth r cla ilicati n of compound within th divi i n de cribed above is based upon th typ and number of th fun tional group which they contain. At this lev I it i n e ary t di tingui h bet n (i) r istry c mpound , and (ii) deri ti of r gi try comp und .
s- ;:; . a :- n 5 · (;; .... ("") - n~?f:T oOl»; 5'~ ~ ~ ~ - ~ ~ ~ ~ ~." 5 ~ 31»oO"'~:S:;'ri 3a.g~ ~ n ~ n~ ~ ~'<: ~~oS:-:3~O ~.~ !. >: ~ . ~ og-()Qn_J5oc~n Vl:-g~ ~ ~c;./.)".-.; :r g ~ n - (5 I n 5'~ 6 g. ~ 0=;-0' '<~",oi' n f P >(;;' 5" a . ~ OQ ..,-, 0=1;;0$ g 3 B :r~.:,. :r n ...... 0 :i' _. - e;. _ ll> n ("") (;n" 3 ;.0;- - Q" ~ Cn::l::T o ::J (11 ~ 0' :r a -6' (; n g. 3 g Q:; 0 • ::1"'0"'0<: C U'J 6l -:!..,:rn",::s nn ..,n 0'1:1 0" -030 - ........ ono -I»-:::,nO -13",1»-0- 3 .., OQ goo (JQ - (JQ I» ::s '" C n • n s: c . ~ S' ji" 0 0 - ::s (') 0 l» o ~ - con 0 '< -- - - . l» o .., (J) ::s \\.... ,.. c..., - c ... '0 ::1 (ii'::-: Ilol ... (J) ::I ... 0 ::s- '" n .. i=)' 0. ::s 0" _ . '" c: c 3 g ::.::3 (') - C 0- 0. 0 & - - . a n :::- .. n - '" _ 0 :::J 'f!1 _ ::r - . 0 0. ... B----- Table 2. Registry Co m pou nds in the Beilstein Ha ndbook - Volume Numbers . Functional Group s Acyclic Isocyclic Heterocyclic compounds C omp ou nds Co mpounds Heteroa tom : Type and Nu mber (n) All Other Oxygen Only Nitrogen On ly Types of n=1 n =1 n=2 2 ~ 3 Heterocycle Compounds Without Funct ional Group s 5 20 23 -oH Hydroxy-Compound s 6 17 1 Oxo-Compound s 21 24 7 =0 Hydroxy-Oxo-Compound s 8 z Carboxylic Acids 9 2 o ;:;' Carboxylic Acids + Hydroxy- and Oxo-Functions en 3 10 0' ... - S0 2 H.- S0 3 H.- S e02 H .- Se0 3 H.- T e02 H 11 19 25 26 27 C en ... o Monoamine s 12 en -NH 2 Polyamines and Hydrox y-Amines 13 18 22 Amines + Other Functional Groups 14 - N H(OH). - N(OH)2' - NHN H2 4 15 -N =NH. - N=N+ . - N H- N=O. - N H- N0 2• Polynitrogen Functional Group s * 16 Compounds Containing C-M Bonds; M=P, As, Sb, Bi; Si, oe, Sn, Pb; B, Al and Other Metals. x • Functional groups containing three or more nitrogen atoms, substituted by - H, - OH and =0 only.
XIV Notes for Users (i) Reg' try compounds are defined as hydrocarbons or heterocycles which bear either no functional groups (i. e. parent compounds), or one or more of the functional groups listed in Table 2, bonded to carbon atoms; no carbon atom, however, may bear more than one functional group *. Three further restrictions apply to heterocyclic rings: the ring must contain at least one carbon atom; ring heteroatom may neither be substituted nor bear functional groups; the ring mu t not contain chalcogen atoms other than oxygen as ring heteroatoms. Compound which do not conform to these criteria are classified as derivatives ( below). • In carboxylic acid. the functional group consists only of the - OH and =0 groups . The volum of the Handbook containing the entry for any particular registry corn- und may be id ntified directly from Table 2 on the basis of its skeletal framework nd functional group . For compounds containing two or more different groups, th Principle of Lat t Sy tematic Entry is again applied, and each compound is ifi 1 und r th functional group which app ar lowest in the list in Table 2. pi, hydro ybenzen ulfonic acid i cia ified as an isocyclic sulfonic acid li hydro yeompound (Vol. 6). In some cases, e.g. not n i.sc:)C}'clic IUUlUl::ll, th nd type of functional group is important to determine in th c m und will d aIt with; thu benzeneI,4diamine (without of fun . n I r up) i in Vol. 13, whereas 4aminobenzoic acid is of gi try compound are giv n below: 0 t}o 0 H 0 I c I C l-CO-DH H • III IV
Recommend
More recommend