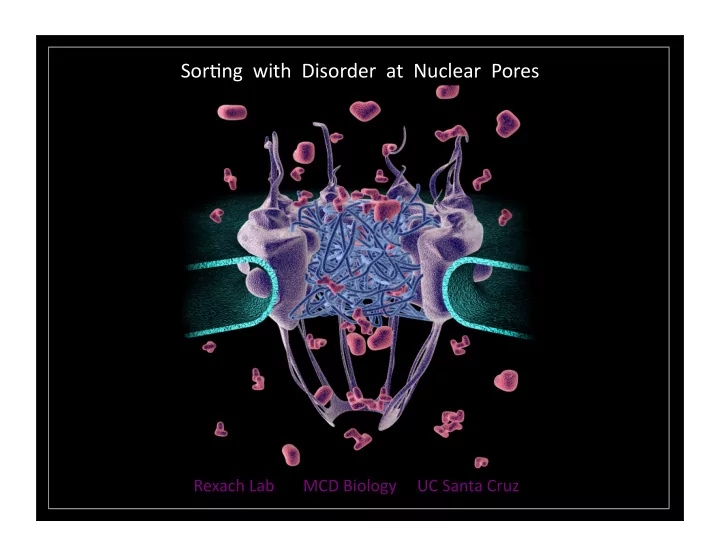

Sor$ng with Disorder at Nuclear Pores Rexach Lab MCD Biology UC Santa Cruz
Nucleocytoplasmic traffic across the nuclear envelope The nuclear envelope is a barrier Thousands of proteins and RNP’s must cross the nuclear envelope every second Nuclear Import Nuclear Export ribosomal proteins transcrip$on factors ribosome subunits mRNA replica$on factors tRNA nucleolar proteins snRNA snRNA rRNA rRNA viral RNP’s viral genomes 2
Nuclear Pores Are Gated by a Molecular Sortase: the Nuclear Pore Complex Sorts based on size , Allows facilitated transport via hydrophobicity & charge karyopherins and signals >3 nm NES ‐ ‐NLS impor$n expor$n >30 kDa Nuclear envelope Chroma$n NES ‐ Ran ‐NLS expor$n < 30 kDa GTP GTP Ran < 3 nm ‐NLS GTP What is the structure of the sortase? impor$n 3
Nucleocytoplasmic transport machinery in S. cerevisiae Nuclear Pore Complex Nucleoporins Karyopherins Impor$ns Expor$ns Non FG Nups FG Nups Nup82 Kap95•Kap60 Crm1 Nup1 Nup84 Kap104 Cse1 Nup2 Nup85 Kap121 Kap120 Nup42 Nup120 Kap123 Los1 Nup49 Nup133 Mtr10 Mex67•Mtr2 Nup53 cNup145 Nmd5 Nup57 Nup157 Sxm1 Nup59 Nup170 Pdr6 Nup60 Nup188 Nup100 Kap114 Nup192 Nup116 N`2 Sec13 nNup145 Seh1 Nup159 Cdc31 Nsp1 Nic96 Transpor$ns Msn5 Ran GTPase and effectors POMs Pom34 Pom152 Ndc1 What is the structure of FG nups? 4
Structural analysis of FG domains of Nups beta random alpha amino acids 500 0 1,000 sheet coil helix N C Nup159 7% 3% 90% Nup100 4% 1% 95% Nup57 4% 1% 95% FG Nups Nsp1 2% 3% 95% Nup1 6% 4% 90% Nup2 13% 7% 80% non FG Nup Nup85 50% 15% 35% 5
The FG domains of nucleoporins are intrinsically‐disordered and form a meshwork of unfolded polypep$des ~150 FG Nups Advantage of having unfolded proteins in the NPC conduit? Func$onal > can bind many proteins simultaneously, karyopherins > have fast molecular on/off rates to “facilitate” rapid diffusion Structural > form a porous filamentous meshwork that restricts free diffusion > form flexible meshwork that accommodates par$cles of all shapes/sizes 6
Rexach Lab MCD Biology UC Santa Cruz Jus$n Yamada Alison Calestagne‐Morelli Hans Huang Gisela Colón Collaborators: Modeling: M. Colvin & Josh Phillips (UC Merced), E. Lau (LLNL) Spectroscopy: K. Krishnan (UC Davis) Polymer Physics: A. Gopinathan (UC Merced) Protein Structure: V. Uversky (IU) Single Molecules: P. Hinterdorfer (Austria)
Recommend
More recommend