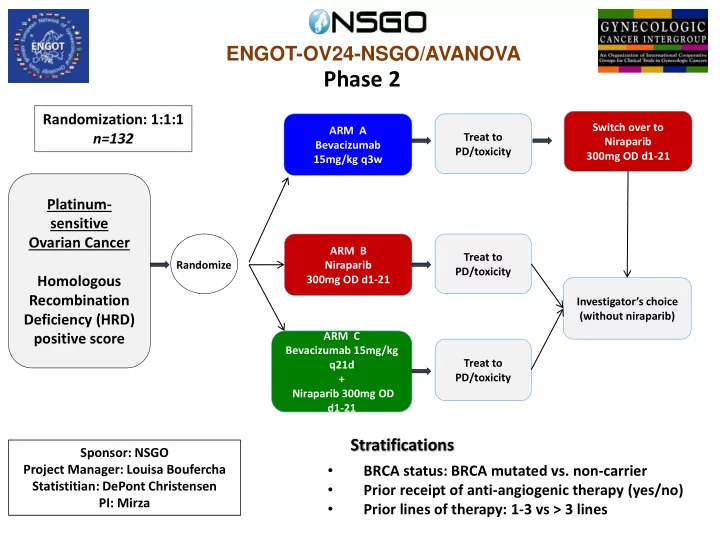

ENGOT-OV24-NSGO/AVANOVA Phase 2 Randomization: 1:1:1 Switch over to ARM A n=132 Treat to Niraparib Bevacizumab PD/toxicity 300mg OD d1-21 15mg/kg q3w Platinum- sensitive Ovarian Cancer ARM B Treat to Randomize Niraparib PD/toxicity Homologous 300mg OD d1-21 Recombination Investigator’s choice (without niraparib) Deficiency (HRD) ARM C positive score Bevacizumab 15mg/kg Treat to q21d PD/toxicity + Niraparib 300mg OD d1-21 Stratifications Sponsor: NSGO • Project Manager: Louisa Boufercha BRCA status: BRCA mutated vs. non-carrier Statistitian: DePont Christensen • Prior receipt of anti-angiogenic therapy (yes/no) PI: Mirza • Prior lines of therapy: 1-3 vs > 3 lines
ENGOT-OV24-NSGO/AVANOVA (Phase 2) - Trial Status Country Sites PI Submission status SIV Randomized • Rigshospitalet Mansoor R. Mirza (NC) CA: Approved: 18.12.2015 03.12.2015 DK 3 • EC: Approved: 01.03.2016 Herlev Trine Juhler-Nøttrup 30.03.2015 2 Odense Jørn Herrstedt 08.03.2016 - Aarhus Ranva Hassel 19.08.2016 - Aalborg Bente Lund 16.12.2015 3 • Tampere Johanna Mäenpää (NC) CA: Approved: 19.07.2016 30.09.2016 FI - • EC: Approved: 06.06.2016 TBD - Kuopio Maarit Anttila Turku Sakari Hietanen TBD - • Haukeland Line Bjørge (NC) CA: Approved: 29.09.2016 TBD NO - • EC: Approved: Oct 2016 Stavanger Bent Fiane TBD - • Lund Susanne Malander (NC) CA: Approved 22.04.2016 16.09.2016 SE - • EC: Approved: 22.03.2016 Linköping Per Rosenberg 26.09.2016 - Sahlgrenska Maria Dimoula 16.09.2016 (web-based) - On site monitoring visit pending Uppsala Hanna Dahlstrand 16.09.2016 (web-based) - On-site monitoring visit: 11.11.2016 (Planned) • MGH Michael Birrer (NC) Hard copies were submitted by GSO to FDA and TBD US - were received the 09.09.2016. Huntsman Cancer Theresa Werner • TBD Re-submission to FDA done 20.09.2016. - Institute • The 16.09.2016 Myriad submitted the risk determination letter and the acknowledgement letter to FDA. It was received on 19.09.2016. • Reply with comments received from FDA on 05.10.2016.The IND was clinically reviewed and following a response were send to FDA to answer their criticisms. Currently waiting for a response from FDA. Total 8
A randomized double-blind placebo-controlled phase II trial of first-line combination chemotherapy with Nintadenib for patients with advanced or recurrent endometrial cancer ENGOT-EN1 / FANDANGO Randomization: 1:1 n=148 ARM A Treat to Endometrial Carboplatin + Paclitaxel PD/toxicity + Cancer Nintedanib Investigator ’ s Stage 3 Randomize choice (without or niraparib) Stage 4 or First relapse ARM B Treat to Carboplatin + Paclitaxel PD/toxicity + Placebo Sponsor: NSGO Stratifications • stage of disease Project Manager: Kicki Jederud (stage 3 vs. stage 4 vs. recurrent disease) • Prior adjuvant chemotherapy Statistitian: DePont Christensen (yes/no) • Disease status PI: Mirza (no macroscopic dis vs. macroscopic dis) mansoor@rh.regionh.dk
GROUP NATIONAL COORDINATOR No. SITES SUBMISSION STATUS SIV performed/planned 4 NSGO Mansoor Raza Mirza, Copenhagen CA Approved 3 SIVs performed Denmark EC Approved 3 NSGO Johanna Mäenpää, Tampere CA Pending Planned Oct/Nov 2016 Finland EC Approved 1 NSGO CA Approved Gunnar Kristensen, Oslo Planned Oct 2016 Norway EC Pending NSGO Per Rosenberg, Linköping CA Approved 4 Planned Oct/Nov 2016 Sweden EC Approved Dr Jalid Sehouli, Charité Campus Virchow- CA Approved NOGGO 12 Planned Nov 2016 Klinikum Berlin EC Approved CA Submitted 30 th Sep 2016 Dr Sevilay Altintas UZ Antwerpen BGOG 6 EC Submitted 4 th Oct 2016 CA Submitted 28 th Sep 2016 Dr Dominique Bertron-Rigaud GINECO 11 EC Submitted 29 th 2016 ICO Centre René Gauducheau Saint-Herblain Total 41 mansoor@rh.regionh.dk
A randomized, double-blind, placebo-controlled, phase II trial of Palbociclib in combination with Letrozole versus Placebo in combination with Letrozole for patients with Estrogen receptor Positive advanced or recurrent Endometrial cancer. ENGOT-EN3-NSGO/PALEO Randomization: 1:1 Sponsor: NSGO N=78 ARM A Endometrial Cancer Letrozole, 2.5mg d 1-28 every 28 days Placebo 125mg d 1-21 every 28 days Primary stage 4 or Until progression relapsed disease Randomize ARM B ER positive Letrozole, 2.5mg d 1-28 every 28 days endometrioid Palbociclib 125mg d 1-21 every 28 days adenocarcinoma Until progression Stratification: • Number of prior lines of therapy (primary advanced disease vs. 1 st relapse vs. ≥2 relapses) Sponsor: NSGO • Measurable vs. evaluable disease Project Manager: Joan Løhndorf • Prior use of MPA/Megace (prior MPA/Megace use capped to a Statistitian: DePont Christensen maximum of 50%) PI: Mirza
GROUP NATIONAL COORDINATOR No. SITES SUBMISSION STATUS NSGO 3 Mansoor Raza Mirza, Copenhagen CA Approved EC Approved Denmark NSGO 3 Annika Auraen, Tampere CA planned submission mid October EC planned submission mid October Finland NSGO 2 CA planned submission start November Line Bjørge, Bergen EC planned submission start November Norway NOGGO CA planned submission during November 5 Jalid Sehouli, Berlin EC planned submission during November Germany MITO CA planned submission during November 6 Giovanni Scambia, Rome EC planned submission during November Italy GEICO CA planned submission during November 5 Dr. Cesar Mendiola, HU 12 de Octubre EC planned submission during November Spain Total 24
NSGO-OV-UMB1: A Phase 2 Umbrella Trial in Recurrent Ovarian Cancer Cohort A Simon 2-stage OX40 + Durvalumab OX40 Coordinating Group design for each SGCTG (days: 0 – 28) (days: 29 – PD) cohort Cohort B Treatment until Relapsed ovarian progression disease cancer OX40 + CTLA4 OX40 Coordinating Group PMHC (days: 0 – 28) (days: 29 – PD) Cohort C CD73 + Durvalumab CD73 Coordinating Group NSGO (days: 0 – 28) (days: 29 – PD) Days: 0 28 56 140 biopsy biopsy biopsy Day: < 0 Day: 28 Day: 56 CT, blood, CT, blood, CT, blood, serum serum serum samples samples samples mansoor@rh.regionh.dk
Recommend
More recommend