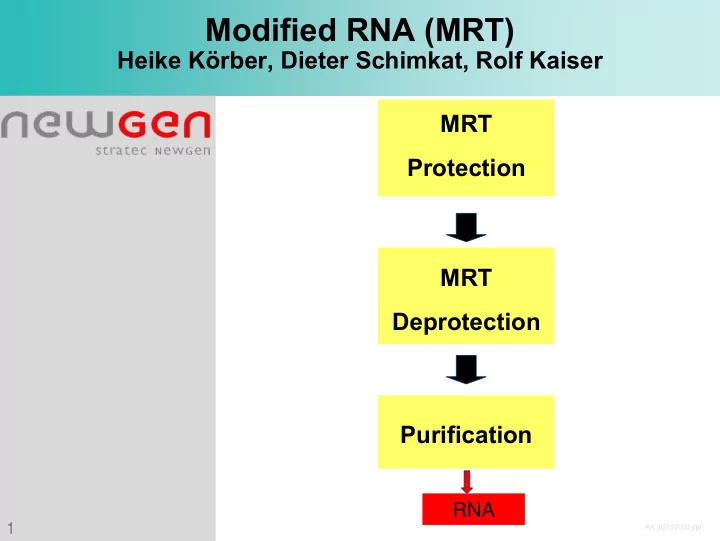

Modified RNA (MRT) Heike Körber, Dieter Schimkat, Rolf Kaiser MRT Protection MRT Deprotection Purification RNA 1 AK_02122003.ppt
Analysis MRT-RNA The mechanism of MRT O O O O NH NH NH NH O O O O N N N N O O O O M O O O O OH OH MRT protection leeds to acetylation of all 2`-OH groups of the RNA molecule 2 AK_02122003.ppt
Viral analysis Assays - HIV-1 Experimental setup: dilutions of HIV-1 in human plasma from 0 – 50 000 copies / ml were prepared with MRTpellet and purified with HXA-Beads + DyeEx The samples were analysed in COBAS Amplicor HIV COBAS Result: Amplicor MRT gives comparable results to UltraSense + HXA-Beads log copies / ml 100000 100000 UltraSense protocol 10000 10000 test series A test series B 1000 1000 MRTpellet protocol 100 100 test series A test series B 10 10 � � � � 1 1 MRT - 50 000 US - 50 000 MRT - 5000 US - 500 US - 5000 MRT - 500 HIV: MRT - 50 US - 50 US - 50 MRT - 0 US - 0 MRT - US - MRT - MRT - US - MRT - US - MRT - US - Retrovirus: ss RNA, enveloped � target too low 3 AK_02122003.ppt
Viral analysis Assays - HIV-1 Experimental setup: Human samples with 100 – 10 000 copies HIV / ml were prepared with Qiagen and MRT + Silica-Beads The samples were analysed in COBAS TaqMan HIV COBAS TaqMan Result: + MRT gives comparable results to Qiagen, but for MRT in low Silica-Beads concentrations one sample failed and one IC failed log copies / ml Qiagen protocol 1000000 MRT protocol 100000 + Silica-Beads 10000 MRT protocol invalid IC 1000 MRT – invalid 100 no target 10 1 HIV: MRT 10 000 MRT 100 Qiagen 10 000 MRT 1 000 Qiagen 1 000 Qiagen 100 Retrovirus: ss RNA, enveloped 4 AK_02122003.ppt
Viral analysis Assays - ECHO-12 Light Cycler Assay (Roche) ECHO-12 analysis in human plasma Overview HXA: Sensitivity +++ viral analysis Reproduction +++ Stability +++ (50 days) ECHO-12 Snap: Sensitivity +++ Reproduction ++ (Qiagen reference � ) Stability ++ (4 weeks) ECHO-12 analysis in whole blood Snap: Sensitivity + � Uni Köln � Non-Infectivity ECHO-12: MRT treated viruses are not pathogen Entero virus: ss RNA, non enveloped 5 AK_02122003.ppt
Viral analysis Assays - ECHO-12 Experimental setup: Enterovirus was spiked into whole blood with 10000 and 100000 copies / ml from a quantified cell culture stock. The samples were treated with MRT and purified with Silica-Beads. ECHO-12 No reference is available! Analysis was carried out in a specific Roche Light Cycler Assay. Light Cycler Result: from whole blood MRT gives lower yields, than the input of virus, but within the same range log copies / ml input copies / ml 1000000 MRT + Silica-Beads 100000 10000 1000 100 10 ECHO-12: 1 0 0 0 0 0 0 T . . 0 T R 0 T T 0 R 1 M R R 1 M Entero virus: t M M u t u p p n ss RNA plus orientated, n I I non enveloped 6 AK_02122003.ppt
Viral analysis Assays - ECHO-12 Experimental setup: Enterovirus was spiked into plasma with 100 000 copies / ml, the plasma was treated in different ways and stored at RT, the samples were purified at the designated time points. ECHO-12 Analysis was carried out in a specific Roche Light Cycler Assay. Result: Light Cycler MRT is comparable to Qiagen and RNAlater, lysed virus degrades after one week, the virus is stable in plasma all the time Stability Test log copies / ml ⇒ pure plasma without 1000000 treatment ⇒ treated with Qiagen lysis 100000 buffer ⇒ treated with RNAlater 10000 ⇒ protected with MRT purified with HXA-Beads 1000 + Microcon 100 10 ECHO-12: 1 0 5 10 15 20 25 30 35 40 45 50 days at room Entero virus: temperature ss RNA, non enveloped 7 AK_02122003.ppt
Viral analysis Assays - HSV-1 Experimental setup: 200 µl human plasma were spiked with cultivated HSV-1. Samples were treated according to the QIAamp DNA Blood Mini Kit (Qiagen) and with MRT, purified with HXA-Beads + Microcon. HSV-1 Analysis was carried out in a specific Roche Light Cycler Assay. Light Cycler Result: + MRT gives comparable or better results than the reference method. HXA-Beads crossing points 11 Reference 9 MRT – HXA-Beads 7 5 3 1 HSV: herpes virus: ds DNA, enveloped 8 AK_02122003.ppt
MRT + Virus Inaktivierung Experimental setup: cultivated human fibroblasts were incubated with supernatant of cultivated human fibroblast, MRT treated � negative control non infectivity of supernatant of HSV-1 infected cultivated human fibroblasts � positive control primary samples supernatant of HSV-1 infected cultivated human fibroblasts, MRT treated � sample treated with MRT Result: MRT inactivates HSV-1. negative control positive control MRT inactivated HSV Qualitative PCRs were carried out from all culture supernatants: HSV: � negative control shows no amplification � positive control shows amplification Herpes Virus: � MRT sample shows amplification (DNA!) ds DNA, enveloped 9 AK_02122003.ppt
MRT + Virus Inaktivierung Experimental setup: cultivated green monkey cells were incubated with supernatant of cultivated green monkey cells, MRT treated � negative control non infectivity of supernatant of ECHO-12 infected cultivated green monkey cells � positive control primary samples supernatant of ECHO-12 infected cultivated green monkey cells, MRT treated � sample treated with MRT Result: MRT inactivates ECHO-12. negative control positive control MRT inactivated ECHO-12 ECHO-12: Qualitative PCRs were carried out from all culture supernatants: � negative control shows no amplification Entero virus: � positive control shows amplification ss RNA plus orientated, � MRT sample can be amplified after MRT Deprotection protocol non enveloped 10 AK_02122003.ppt
MRT + Virus Inaktivierung Experimental setup: cultivated human donor lymphocytes were incubated with supernatant of cultivated human donor lymphocytes, MRT treated � negative control non infectivity of supernatant of HIV-1 infected cultivated human donor lymphocytes � positive control primary samples supernatant of HIV-1 infected cultivated human donor lymphocytes, MRT treated � sample treated with MRT Result: MRT inactivates HIV. negative control positive control MRT inactivated HIV-1 p24 ELISA was carried out from all culture supernatants: HIV: � negative control shows no p24 � positive control shows p24 Retrovirus: � MRT sample shows no p24 ss RNA, enveloped 11 AK_02122003.ppt
MRT + Virus Inaktivierung Humane Spender Lymphozyten wurden inkubiert mit Inaktivierung � MRT behandelten HIV Kulturen infektiöser humaner Proben ⇒ Spender Lymphozyten zeigen keine Symptome einer HIV Infektion HIV ⇒ p24 ELISA ist negativ 12 AK_02122003.ppt
HIV1 - - Lymphocytenkultur Lymphocytenkultur HIV1 13 AK_02122003.ppt
Lymphocytenkultur Lymphocytenkultur 14 AK_02122003.ppt
HIV produzierende Lymphozyten 15 AK_02122003.ppt
Recommend
More recommend