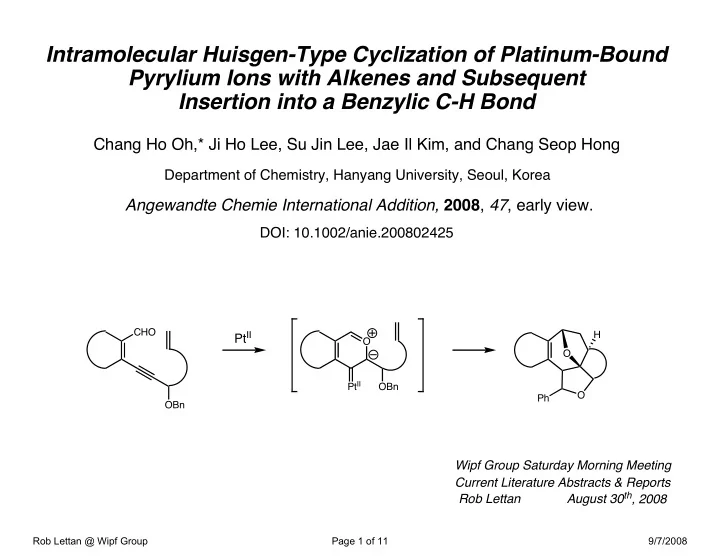

Intramolecular Huisgen-Type Cyclization of Platinum-Bound Pyrylium Ions with Alkenes and Subsequent Insertion into a Benzylic C-H Bond Chang Ho Oh,* Ji Ho Lee, Su Jin Lee, Jae Il Kim, and Chang Seop Hong Department of Chemistry, Hanyang University, Seoul, Korea Angewandte Chemie International Addition, 2008 , 47 , early view. DOI: 10.1002/anie.200802425 CHO Pt II H O O Pt II OBn O Ph OBn Wipf Group Saturday Morning Meeting Current Literature Abstracts & Reports August 30 th , 2008 Rob Lettan Rob Lettan @ Wipf Group Page 1 of 11 9/7/2008
Oxabicyclo[3.2.1]octanes in Natural Products O O OH O O O Me Me O HO 2 C N O H O OH O O H Me Me platensimycin corianlactone broad spectrum Gram-positive antibiotic no remarkable inhibition towards K 562 cells Synthesized by: cytotoxic, IC 50 > 50 µ g/mL Nicolaou (2006, racemic) (2007, 2008 asymmetric) Snider (2007, formal, racemic) E. Lee (2008, formal) RO OR HO R Me O RO Me OH Me H HO RO Me OR RO R bruguierol C showed moderate activity against Gram-positive Taxane diterpenoids and Gram-negative bacteria anti-cancer Synthesized by: Jennings (2007) Ramana (2007, bruguierol A) Rob Lettan @ Wipf Group Page 2 of 11 9/7/2008
Strategies for the Construction of 7-Membered Carbocycles: [6C + 1C] Approach O O MeO OH CH 2 =S(O)Me 2 TFA MeO CO 2 Me OMe OMe MeO MeO OMe MeO OMe MeO MeO OMe MeO MeO MeO MeO MeO NHAc CO 2 Me MeO CO 2 Me 92% MeO MeO MeO OMe O OMe MeO OMe OMe O (±)-colchicine Evans, D. A., et al. J. Am. Chem. Soc. 1981 , 103 , 5813-5821. MeO O MeO MeO H CO 2 Me H CO 2 Me H CO 2 Me H Rh II DBU O Me Me Me Me 65% Me Me N 2 Me O Me ODEIPS ODEIPS ODEIPS H H O MeO OMe O MeO OMe MeO OMe O O harringtonolide Mander, L. N., et al. Aust. J. Chem. 2000 , 53 , 819-830. Rob Lettan @ Wipf Group Page 3 of 11 9/7/2008
Strategies for the Construction of 7-Membered Carbocycles: [5C + 2C] Approach Me OAc OH O Me O Me Me Me Me H H OH O DBU Me H O OAc O OAc MeCN 79% OH O AcO O HO OTBS OTBS OTBS OH phorbol Wender, P. A., et al. J. Am. Chem. Soc. 1997 , 119 , 7897-7898. This approach was also used by many others in natural product syntheses: P. Magnus (guanacastepene, taxanes), J. E. Baldwin (cordytropolone: alkyne trap), and Snider (cartorimine: unsat. ester) Review: Wright, D. L., et al. Chem. Eur. J. 2006 , 12 , 3438-3447. H H H McMills, M. C., et al. Tet. Lett. Rh 2 (OHex) 4 1994 , 35 , 8311-8314. O O H 85% Dauben, W. G., et al. J. Org. O O R Chem. , 1993 , 58 , 7635-7637. H H H O O R N 2 R tigliane skeleton Me Me O Me Me Me Me Me Me • Me Me [Rh(CO) 2 Cl] 2 Me Ln Rh 90% RhLn Me H Me Me Me Me Me Me H Me H H Me Me BnO Me OBn Me HO OBn BnO (+)-aphanamol I Wender, P. A., Zhang, L. Org. Lett. 2000 , 2 , 2323-2326. Rob Lettan @ Wipf Group Page 4 of 11 9/7/2008
Strategies for the Construction of 7-Membered Carbocycles: [4C + 3C] Approach O Me Me Me Me Cl Cl O Me Zn-Cu O O O O OH 70% H H H H O O O O O Me O O OH Me Cl Cl OH O O erinacine C Wright, D. L., et al. J. Org. Chem. 1999 , 1 , 1535-1538 OTMS TMSO OMe MeO MeO MeO NHBoc MeO NHBoc OMe colchicine MeO NHBoc 45% MeO TMSOTf O OMe MeO OMe O O OMe O OMe Cha, J. K., Lee, J. C. Tetrahedron 2000 , 56 , 10176-10184. MeO MeO MeO 75 °C MeO NHAc O O O O MeO MeO O 73% MeO OMe O OMe CO O O O O OMe (±)-colchicine Boger, D. L., Brotherton, C.E. J. Am. Chem. Soc. 1986 , 108 , 6713-6719. Rob Lettan @ Wipf Group Page 5 of 11 9/7/2008
Strategies for the Construction of 7-Membered Carbocycles: Alternative Approaches O O O 1. (PPh 3 ) 3 RhCl, H 2 TMS Me O O 2. n Bu 4 F Me O N N N O TMS 3. CH 2 O, Na(CN)BH 3 Rh 2 (OAc) 4 Me Me N 73% O 70%, 3 steps O TMS Me O ferruginine N 2 Davies, H. M. L., et al. J. Org. Chem. 1991 , 56 , 5696-5700. Me O O Me H H O O O O O Rh 2 ( S -DOSP) 4 H 2 , ClRh(PPh 3 ) 3 140 °C N 2 76% 85% Me Me H Me H Me Me 90% ee Me Me Me Me Me tremulenolide Davies, H. M. L., Doan, B. D. Tet. Lett. 1996 , 37 , 3967-3970. O PMP O O PMP O O PMP O 1. SmI 2 CHO O AcO 2. PhSeBr O O O OH h � 3. m CPBA Me 37%, 76% Me Me 3 steps Me Me Me Me Me Me Me guanacastepene Sorenson, E. J., Shipe, W. D. Org. Lett. 2002 , 4 , 2063-2066. Rob Lettan @ Wipf Group Page 6 of 11 9/7/2008
Transition-Metal Catalyzed Cyclization of Pyrylium Ions with Alkenes/Alkynes Me Me Me Me Me Me OEt O 20 mol% i -Pr W(CO) 5 ·THF O OEt O C-H insertion O OEt OEt H OCH 2 CH 3 H THF, rt Me —W(CO) 5 ·THF O H W(CO) 5 W(CO) 5 76% Iwasawa, N. et al. J. Am. Chem. Soc. 2001 , 123 , 5814-5815. Ph CHO 10 mol% AuCl 3 O O Ph Ph Ph Ph Ph AuCl 3 AuCl 3 O Ph 96% yield >99:1 regioselectivity Yamamoto, Y., et al. J. Am. Chem. Soc. 2002 , 124 , 12650-12651. Rh O Rh [Rh(COD)Cl] 2 , dppp [5+2] H 2 O CHO TsN 80 °C, 8 h TsN O TsN O O TsN H H 87% Oh, C. H., et al. Chem. Commun. 2005 , 4429-4431. Rob Lettan @ Wipf Group Page 7 of 11 9/7/2008
Intramolecular Platinum Catalyzed Cyclization of Pyrylium Ions with Alkenes and Subsequent C-H Insertion CHO H H 5 mol% Pt II O O O toluene 120 °C, 1h H Pt II 2a OBn Pt II O O Ph OBn H Ph H PhO 2 C Catalyst 2a 3a 4a H 35% — PtCl 2 25% 3a — PtCl 4 20% 40% — — Pt(PPh 3 ) 4 20% O — 4a — PtCl 2 (PPh 3 ) 2 77% — PtCl 2 (dppe) 61% 10% Rob Lettan @ Wipf Group Page 8 of 11 9/7/2008
Mechanistic Proposal CHO H H Pt II O O O Pt II OBn Pt II Pt II O O OBn Ph Ph PhOCO H H H H a O O b O 3a H Pt a Pt Pt O O O Ph Ph 1. NaOH Ph H 2. PCC b O H H H O O H 4a 2a H O Ph Rob Lettan @ Wipf Group Page 9 of 11 9/7/2008
Reaction Scope SM Product SM Product CHO CHO H H O O 80% 74% H H O O Ph Ph OBn OBn CHO H CHO H O O 66% 88% H H O Ph O Ph OBn OBn O PhOCO CHO CHO H 80% 86% OBn BnO BnO CHO OH O H H OTBS PDC O O H H O O Ph Ph OBn 76% Rob Lettan @ Wipf Group Page 10 of 11 9/7/2008
Summary Methods for the synthesis of 7-membered carbocycles are necessary due to their prevelance in natural products. Several tactics for the formation of 7-membered carbocycles have been developed, including strategies that employ, [6C + 1C], [5C + 2C], [4C + 3C], and other alternatives. Oh and coworkers have published a platinum-catalyzed cyclization of pyrylium ions with alkenes to give a tetracyclic platinum-carbene complex, which immediately undergoes insertion into a benzylic C-H bond to give a highly complex ring system. The power of transition metal-catalyzed pyrylium ion formation and subsequent cyclization has been demonstrated to access various multi-cyclic scaffolds, and lends to possibility of utilization of these methods for the future development of other sophisticated carbon frameworks. Rob Lettan @ Wipf Group Page 11 of 11 9/7/2008
Recommend
More recommend