
HP 5988A EI/CI GC/DIP/MS System uses a quadrupole mass analyzer
Agilent 6850/5973 GC/MSD uses a quadrupole analyzer
ABI Voyager Pro MALDI-TOF MS system
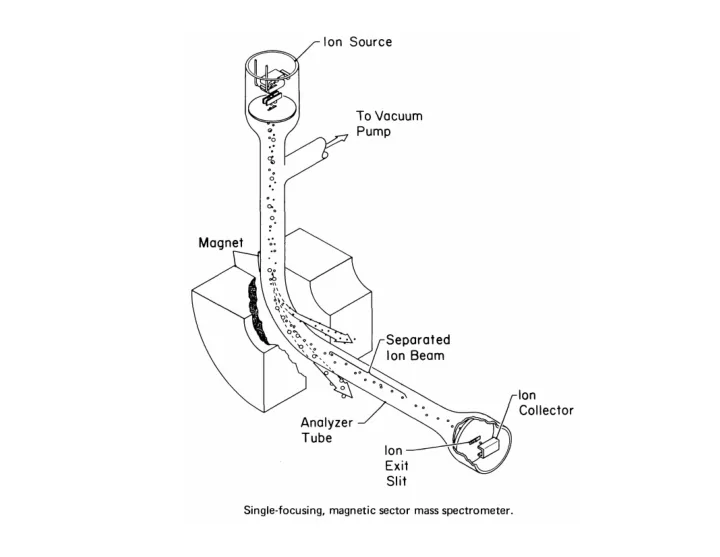
Mass spectrometers weight individual molecules and pieces of individual molecules. Mass spectrometry is useful for • Determining molecular mass < at unit resolution for example, benzene has MF C 6 H 6 MW = 6(12) + 6(1) = 78 g/mol in mass spectrum, observe m/ z M + C = 78 amu • Determining molecular formula < at unit resolution isotopic contributions to (M+1) + C and (M+2) + C allow MF to be deduced < at high resolution mass spectral resolution of <5 ppm allows MF to be identified • Determining structure the gas-phase elimination and rearrangement mechanisms of M + C are diagnostic of < constitution
N O 82 O O O 96 88 Cocaine 80 182 C 17 H 21 NO 4 72 m/z = 303 64 56 48 40 105 32 42 24 303 51 16 198 272 152 8 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300
ION Height 303 M + C 100 -204 C 17 (M+1) + C 19.7 99 (M+2) + C 0.8 -14 N 85 maximum # C in MF: 1.1 x # 20 -64 O 4 x # 18 21 -21 H 21 if C 17 , contribution to (M+1) + C = 1.1(17) = 18.7 M + C 0 remaining (M+1) + C = 20 - 18.7 = 1.3 if C 18 , contribution to (M+1) + C = 1.1(18) = 19.8 remaining (M+1) + C = 20 - 19.8 = 0.2 M + C is odd, so N is in MF, have to choose C 17 for C 17 N 1 remaining (M+1) + C = 1.3 - 0.37 = 0.9 maximum #O in MF: 0.20 y # 0.8 y # 4 (M+1) + C for C 17 NO 4 , remaining (M+1) + C = 0.2 - 0.2 = 0 (M+2) + C The number of amu left corresponds to H’s C 17 H 21 NO 4 relative abundances of isotopes 12 C 1 H 14 N 16 O 35 Cl 79 Br M 100 100 100 100 100 100 13 C 2 H 15 N 17 O M+1 1.1 0.015 0.37 0.04 ----- ----- 18 O 37 Cl 81 Br M+2 ----- ----- ----- 0.20 32 97.3
2-Methyl-3-buten-2-ol 71 OH 100 C 5 H 10 O 90 80 70 43 60 50 40 59 41 30 + M @ 27 20 31 53 15 72 10 86 0 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85
2-Heptanone 43 O 100 71 90 C 7 H 14 O 80 70 60 50 + M @ 40 30 27 114 20 58 10 99 15 86 53 81 0 20 30 40 50 60 70 80 90 100 110 . . . . + . O . O . . . + . O . + . . . . . . + O . O . . O + . + . + . . m/ z = 71 m/ z = 43
3-Bromo-1-propene 41 Br 100 C 3 H 5 Br 90 80 70 60 50 40 30 120 20 26 79 10 15 105 93 0 10 20 30 40 50 60 70 80 90 100 110 120 . . . . Br + . . . . . . + . + . . Br . . . + Br . . . . . + Br . + . . m/ z = 79 m/ z = 41
(C 26 H 19 O 2 ) + + H + m/z = 363.1386 found (corrected): m/z = 363.1804 O O
Calculate the exact mass of (C 26 H 19 O 2 ) + Element Mass C 12.00000000 26(12.00000000) + 19(1.007825035) + 2(15.99491463) H 1.007825035 O 15.99491463 = 363.1385 found (corrected) m/z = 363.1804 the difference is -.0419 amu , which corresponds to a 0.01% deviation from the calculated exact mass this is a difference of 115 ppm, very large compared to the accuracy of the measurement, which is 5 ppm ∆ 15 ppm (C 27 H 23 O) + MF with better matches: 363.1749 (C 24 H 27 O 3 ) + ∆ 43 ppm 363.1960
(C 13 H 16 N 3 O 2 ) + m/z = 246.1239 O + found (corrected): H 3 N m/z = 246.1246 O N 2.8 ppm difference N (best match for this MF) H
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: IR spectrum A Mass spectrum A
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: 2100 cm -1 C / / / / C stretch 3300 cm -1 Csp–H < 3000 cm -1 : stretch Csp 3 –H stretch > 3000 cm -1 : ~1600 cm -1 Csp 2 –Hstretch C=C stretch 910 cm -1 625 cm -1 R 2 C=CH 2 bend RC / / / / CH bend Mass spectrum A Clearly label two features on the IR spectrum indicating the functional group class of product A.
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: 2100 cm -1 C / / / / C stretch 3300 cm -1 Csp–H < 3000 cm -1 : stretch Csp 3 –H stretch > 3000 cm -1 : ~1600 cm -1 Csp 2 –Hstretch C=C stretch 910 cm -1 625 cm -1 R 2 C=CH 2 bend RC / / / / CH bend Mass spectrum A M + A A A A A ) Clearly label the molecular ion (M + of the mass spectrum.
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: 2100 cm -1 C / / / / C stretch 3300 cm -1 Csp–H < 3000 cm -1 : stretch Csp 3 –H stretch > 3000 cm -1 : ~1600 cm -1 Csp 2 –Hstretch C=C stretch 910 cm -1 625 cm -1 R 2 C=CH 2 bend RC / / / / CH bend Mass spectrum A A A M + A A The fragment appearing at mass 51 suggests a loss of what functional group from the molecular ion? 66 - 51 = 15 mass units: C (12) + 3 [H (1)] = CH 3 (15)
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: 2100 cm -1 C / / / / C stretch 3300 cm -1 Csp–H < 3000 cm -1 : stretch Csp 3 –H stretch > 3000 cm -1 : ~1600 cm -1 Csp 2 –Hstretch C=C stretch 910 cm -1 625 cm -1 R 2 C=CH 2 bend RC / / / / CH bend Mass spectrum A A A M + A A Provide a structural formula for product A .
Solvolysis of 3-bromo-3-methyl-1-butyne (M.W. 147) in water at 50 E C for 12 hours gives two products, A and B. The infrared and mass spectra of product A are shown below: 2100 cm -1 C / / / / C stretch 3300 cm -1 Csp–H < 3000 cm -1 : stretch Csp 3 –H stretch > 3000 cm -1 : ~1600 cm -1 Csp 2 –Hstretch C=C stretch 910 cm -1 625 cm -1 R 2 C=CH 2 bend RC / / / / CH bend Mass spectrum A A A M + A A Product B has molecular formula C 5 H 8 O. Provide a structural formula for product B OH
Recommend
More recommend