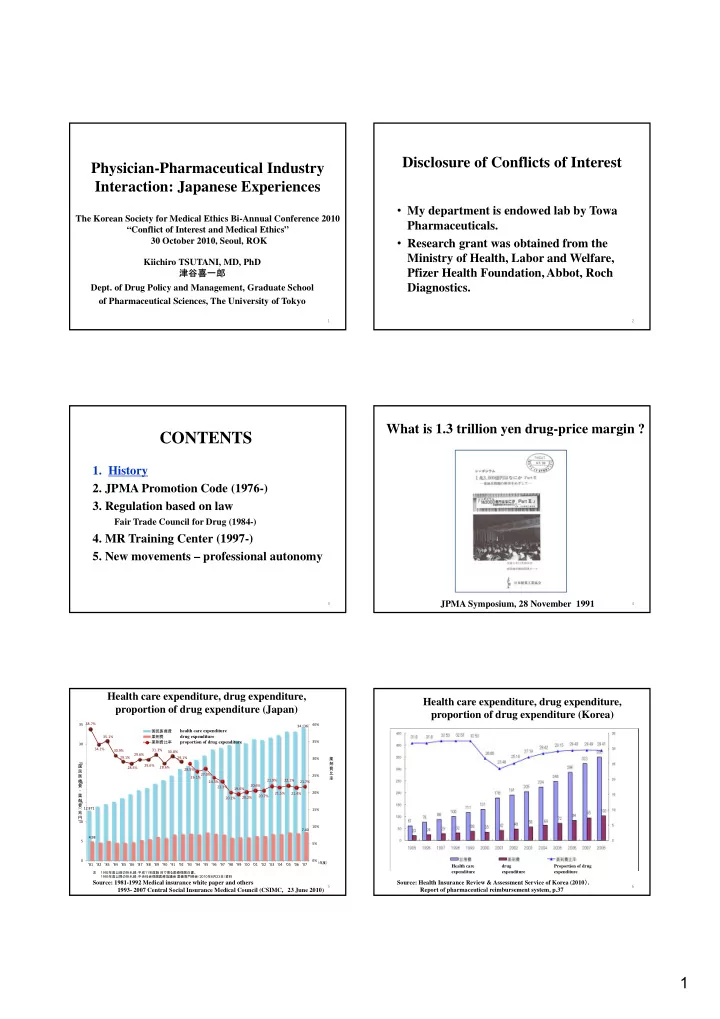

Disclosure of Conflicts of Interest Physician-Pharmaceutical Industry Interaction: Japanese Experiences • My department is endowed lab by Towa The Korean Society for Medical Ethics Bi-Annual Conference 2010 Pharmaceuticals. “Conflict of Interest and Medical Ethics” • Research grant was obtained from the 30 October 2010, Seoul, ROK Ministry of Health, Labor and Welfare, Kiichiro TSUTANI, MD, PhD 津谷喜一郎 Pfizer Health Foundation, Abbot, Roch Diagnostics. Dept. of Drug Policy and Management, Graduate School of Pharmaceutical Sciences, The University of Tokyo 1 2 What is 1.3 trillion yen drug-price margin ? CONTENTS 1. History 2. JPMA Promotion Code (1976-) 3. Regulation based on law Fair Trade Council for Drug (1984-) 4. MR Training Center (1997-) 5. New movements – professional autonomy JPMA Symposium, 28 November 1991 3 4 Health care expenditure, drug expenditure, Health care expenditure, drug expenditure, proportion of drug expenditure (Japan) proportion of drug expenditure (Korea) 38.7% 35 40% 34.136 国民医療費 health care expenditure 35.1% 薬剤費 drug expenditure 薬剤費比率 proportion of drug expenditure 35% 30 34.1% 31.3% 30.9% 30.8% 29.6% 29.1% 29.1% 30% 薬 25 剤 国 29.6% 28.6% 28.5% 費 28.5% 民 比 26.1%27.0% 医 25% 率 療 21.9% 22.1% 24.5% 21.7% 20 費 20.6% 23.3% 19.6% ・ 20% 21.5% 21.4% 薬 20.7% 20.2% 20.1% 剤 15 費 12.871 ( 15% 兆 円 ) 10 10% 7.40 4.98 5 5% 0 0% (年度) '81 '82 '83 '84 '85 '86 '87 '88 '89 '90 '91 '92 '93 '94 '95 '96 '97 '98 '99 '00 '01 '02 '03 '04 '05 '06 '07 Health care drug Proportion of drug 注 1992 年度以前の折れ線:平成 11 年度版 目で見る医療保険白書。 expenditure expenditure expenditure 1993 年度以降の折れ線:中央社会保険医療協議会 薬価専門部会( 2010 年 6 月 23 日)資料 Source: Health Insurance Review & Assessment Service of Korea (2010 ) . Source: 1981-1992 Medical insurance white paper and others 5 6 1993- 2007 Central Social Insurance Medical Council (CSIMC, 23 June 2010) Report of pharmaceutical reimbursement system, p.37 1
Separation of prescribing and Physicians in health service system dispensing in Japan in Japan and Korea Japan Korea Membership to Voluntary Compulsory medical membership membership association to JMA to KMA Health insurance Non Compulsory physician compulsory “ Mixed payment ” Prohibited No-problem Korean physicians seem to be “quasi civil servant” Source: Annual Report of Japan Pharmaceutical Association(2008-2009) 7 8 Major stake holders affecting physicians- Human resources in health (2008) pharmaceutical industry relation Japan Korea (127 million) (48 million) Number per Number per Pharmaceutical Physician 100,000 100,000 industry Physicians 283,915 224 95,013 198 Pharmacists 249,251 196 58,363 122 Pharmacist Law/Gov. MR 58,000 46 ? ? Source: Ministry of Health, Labor and Welfare (MHLW) of Japan Ministry of Health and Welfare (MHW) of Korea 9 10 Short history (2) 1970s Short history (1) 1960s “Era of Premiums”( 景品) sales Era of “Compensation for discount” Economics status: stable growth Economic status: rapid economic growth 1970 MHW noticed problems in distribution system 1961 Universal health insurance coverage - Drug company pays money to wholesaler for ⇒ Drug market expansion compensation ⇒ Overheated non-price competition - Prohibited “extra” attachment “extra” attachment ( 現品給付 ) - Deletion of four drugs from drug tariff “premiums” offer ( 景品類 ) 1976 JPMA promotion code 1979 MHW regulated “sample”( 試供品) offer “travel invitation” 11 12 2
Short history (3) 1980s CONTENTS Era of Internationalization General economic status: matured ⇒ Control medical expenditure 1. History 1981 Reduction of drug price (18.6%) 2. JPMA Promotion Code (1976-) Cartel by JPMA was accused by Fair Trade Commission 1984Fair Trade Council for Drug ( 日本医薬品公正取引協議会 ) 3. Regulation based on law established Fair Trade Council for Drug (1984-) 4. MR Training (1997-) Early 1980s 5. New movements – professional autonomy Trade conflict between US and Japan American pressure for open and transparent market 1985 Market Oriented Sector Selective (MOSS) Discussions 1989 Japan-US Structural Impediments Initiative (SII) 13 14 JPMA Promotion Code Contents of Promotion Code (JPMA) 日本製薬工業協会 : 製薬協 1. Responsibility of member companies 1976 issued by JPMA, founding member of 2. Responsibility of the top management of member companies IFPMA (1968) 3. Medical Representatives ’ activity standards 4. Production and use of promotional materials and 1967 WHA20.35 ethical criteria advertisements Self-regulation 5. Post-marketing safety control operations and post-marketing - to retain high ethical awareness surveillance - to comply with applicable regulations and 6. Supply of samples 7. Seminars and Study Meetings voluntary standards 8. Gifts - to respond to social trust 9. Provision of cash or its equivalents 1981 IFPMA Code 10.Relations with “the Fair Competition Code of the Ethical 1988 WHO criteria for drug promotion Drug Manufacturing Industry” 11.Promotional activities outside Japan JPMA code has been frequently revised. 15 16 FPMAJ Ethics code (1983) (The Federation of Pharmaceutical Manufacturers’ CONTENTS Association of Japan: FPMAJ, 日本製薬団体連合会 : 日薬連) 1. History JPMA → 2. JPMA Promotion Code (1976-) Generic → 3. Regulation based on law Fair Trade Council for Drug (1984-) 4. MR Training Center (1997-) Kampo → 5. New movements – professional autonomy OTC → 17 18 http://www.fpmaj.gr.jp/sosikizu/index.htm 3
Criminal law case in 2000 Japan Korea KPMA JPMA Asahi Shimbun 18June 2000 Fair Trade Commission Fair Trade Commission Dual Punishment Law Criminal Law Criminal Law “Bribery” charge is for civil servant only i.e. physicians at public hospital 19 20 Possible actions by Fair Trade Council Sentence by the court, on the basis of criminal law for Drug ( 医療用医薬品公正取引協議会 ) Bribery giver (pharm. company) Fine( 罰金 ) : up to 1 mil. yen 1. Delisting from the enrollment in the Code Bribery taker (physician) 2. Imposing Penalty (up to 1 mil. yen) imprisonment: 2 years 3. (Stern Warning 厳重警告 ) with 3 years suspension 4. Warning Medical Ethics Council ( 医道審議会 ) 5. (Caution 注意 ) one year and a half suspension of medical practice of the physician --- Bribery taker 21 22 Fair Trade Council of the Ethical Pharmaceutical Drugs Criticism to FTCD from media Marketing Industry (FTCD) 医療用医薬品製造販売業公正取引協議会 Asahi shinbun 11 January 2001 • Established in 1984 • Industry self regulatory body, supervised by Free Trade Commission (FTC) • Function - to promote of knowledge and understanding of Code - to counsel, guide and dispose of complaints of Code - to investigate of suspected violation of Code Stern Warning - to spread of information concerning “the Act against unjustifiable premiums and misleading representations” and “Too lenient to companies” laws and regulations 23 24 4
Fair Competition Code in Ethical Pharmaceutical Contents of Code (12) Drugs Marketing Industry 1. Purpose and • Objective 2. Definitions to prevent inducement of customers by restricting offers of 3. Principle of restriction on premium offers unjustifiable premiums and misleading representations 4. Examples of restricted premium offers to ensure consumer’s independent and rational choice and 5. Examples of non-restricted premium offers fair competition between businesses entities • “Act against unjustifiable premiums and misleading 6. Restriction on premium offers to ethical pharmaceutical drugs wholesalers representations” (不当景品類及び不当表示防止法) 7. Fair trade council • Authorized by Consumer Affairs Agency and Fair Trade Commission (FTC) ( 公正取引委員会 , 消費者庁 ) 8. Entrepreneurs’ obligation to cooperate • Three documents 9. Investigation of suspected violations - Code (規約 , 12 articles ) , 10. Penalties for violations - Enforcement rules (施行規則 , 6 articles ) 11. Decision on violation - Guidelines (運用基準 , 10 guidelines ) 12. Establishment of enforcement rules 25 26 Contents of Guidelines (11) FTCD action for violation I-1 The principle of premium offers I-2 Donations Yr. II The operation of article 4 of the Code HQ Branch(caution) III-1 Necessary and useful goods and services 2004 2 7(1) III-2 Medical or pharmaceutical information 2005 1 1(1) III-3 Drug samples 2006 0 6(3) III-4 Entrustment of research and studies 2007 1 3(2) III-5 Lecture meetings, etc. of an offerer’s pharmaceutical drugs Ⅳ -1 Small and fair premiums 2008 0 2(1) Ⅳ -2 Social gatherings 2009 0 17(7) Ⅳ -3 Memorial events Source: FTCD(2010) 27 28 Example of “warning” on lecture meeting, 2010 CONTENTS 1. History 2. JPMA Promotion Code (1976-) 3. Regulation based on law Fair Trade Council for Drug (1984-) 4. MR Training Center (1997-) 5. New movements – professional autonomy 29 30 5
Recommend
More recommend