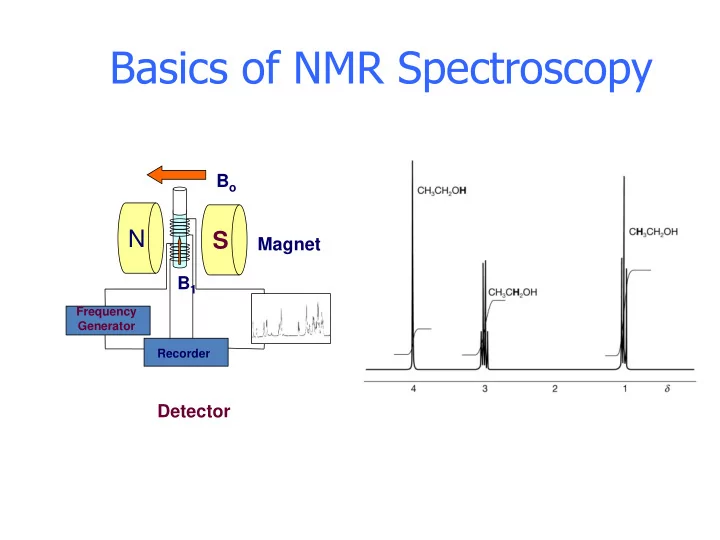

Basics of NMR Spectroscopy B o N S Magnet B 1 Frequency Generator Recorder Detector
Electromagnetic Spectrum
Electromagnetic Spectrum Electronic
Electromagnetic Spectrum Electronic Vibration
Electromagnetic Spectrum Electronic Vibration Rotation
Electromagnetic Spectrum Electronic Vibration Rotation Spin resonance
Nuclear Magnetic Resonance (NMR) Certain nuclei absorb radiofrequencies (electromagnetic radiation) when they are placed in a magnetic field. Essential criterion: spin number ( I ) 0. I = 0 • Even atomic mass & number ( 12 C, 16 O) I = whole integer • Even atomic mass & odd number ( 14 N, 2 H, 10 B) I = half integer • Odd atomic mass ( 1 H, 13 C, 15 N, 31 P) Angular momentum = [I(I+1)] 1/2 h/2 Z-component of angular momentum = m h/2 m = I, (I - 1), (I - 2), … , -I For 1 H: m = 1/2, -1/2
Nuclear Magnetic Resonance (NMR) Certain nuclei absorb radiofrequencies (electromagnetic radiation) when they are placed in a magnetic field. Essential criterion: spin number ( I ) 0. I = 0 • Even atomic mass & number ( 12 C, 16 O) I = whole integer • Even atomic mass & odd number ( 14 N, 2 H, 10 B) I = half integer • Odd atomic mass ( 1 H, 13 C, 15 N, 31 P) Angular momentum = [I(I+1)] 1/2 h/2 Z-component of angular momentum = m h/2 m = I, (I - 1), (I - 2), … , -I For 1 H: m = 1/2, -1/2
Nuclear Magnetic Resonance (NMR) Certain nuclei absorb radiofrequencies (electromagnetic radiation) when they are placed in a magnetic field. Essential criterion: spin number ( I ) 0. I = 0 • Even atomic mass & number ( 12 C, 16 O) I = whole integer • Even atomic mass & odd number ( 14 N, 2 H, 10 B) I = half integer • Odd atomic mass ( 1 H, 13 C, 15 N, 31 P) Angular momentum = [I(I+1)] 1/2 h/2 Z-component of angular momentum = m h/2 m = I, (I - 1), (I - 2), … , -I For 1 H: m = 1/2, -1/2
Nuclear Magnetic Resonance (NMR) Certain nuclei absorb radiofrequencies (electromagnetic radiation) when they are placed in a magnetic field. Essential criterion: spin number ( I ) 0. I = 0 • Even atomic mass & number ( 12 C, 16 O) I = whole integer • Even atomic mass & odd number ( 14 N, 2 H, 10 B) I = half integer • Odd atomic mass ( 1 H, 13 C, 15 N, 31 P) Angular momentum = [I(I+1)] 1/2 h/2 Z-component of angular momentum = m h/2 m = I, (I - 1), (I - 2), … , -I For 1 H: m = 1/2, -1/2
Nuclear Magnetic Resonance (NMR) Certain nuclei absorb radiofrequencies (electromagnetic radiation) when they are placed in a magnetic field. Essential criterion: spin number ( I ) 0. I = 0 • Even atomic mass & number ( 12 C, 16 O) I = whole integer • Even atomic mass & odd number ( 14 N, 2 H, 10 B) I = half integer • Odd atomic mass ( 1 H, 13 C, 15 N, 31 P) Angular momentum = [I(I+1)] 1/2 h/2 Z-component of angular momentum = m h/2 m = I, (I - 1), (I - 2), … , -I For 1 H: m = 1/2, -1/2
The effect of magnetic fields on nuclei For a steady magnetic field B 0 , E = -m B 0 ( m = Magnetic moment = g I ) g = Magnetogyric ratio; g ħ = g l m N ) m N = eħ/2m p = Nuclear magneton = 5.051 x 10 -27 JT -1 g l = Nuclear g factor (Range = -6 to +6), Ĥ = - g B 0 î Considering the field to be along the z-direction, m z = g I z = g m ħ ; E = - m z B 0 = - g m ħ B 0 Different spin states have different energies in the presence of a magnetic field
The effect of magnetic fields on nuclei For a steady magnetic field B 0 , E = -m B 0 ( m = Magnetic moment = g I ) g = Magnetogyric ratio; g ħ = g l m N ) m N = eħ/2m p = Nuclear magneton = 5.051 x 10 -27 JT -1 g l = Nuclear g factor (Range = -6 to +6), Ĥ = - g B 0 î Considering the field to be along the z-direction, m z = g I z = g m ħ ; E = - m z B 0 = - g m ħ B 0 Different spin states have different energies in the presence of a magnetic field
The effect of magnetic fields on nuclei For a steady magnetic field B 0 , E = -m B 0 ( m = Magnetic moment = g I ) g = Magnetogyric ratio; g ħ = g l m N ) m N = eħ/2m p = Nuclear magneton = 5.051 x 10 -27 JT -1 g l = Nuclear g factor (Range = -6 to +6), Ĥ = - g B 0 î Considering the field to be along the z-direction, m z = g I z = g m ħ ; E = - m z B 0 = - g m ħ B 0 Different spin states have different energies in the presence of a magnetic field
N uclear M agnetic R esonance Spin ½ nucleus ( 1 H, 13 C)
N uclear M agnetic R esonance Spin ½ nucleus ( 1 H, 13 C) E = E -E = ½ g ħB 0 – (- ½ g ħB 0 ) = g ħB 0
N uclear M agnetic R esonance Spin ½ nucleus ( 1 H, 13 C) E = E -E = ½ g ħB 0 – (- ½ g ħB 0 ) = g ħB 0 = h L i.e. L = g B 0 / 2
N uclear M agnetic R esonance Spin ½ nucleus ( 1 H, 13 C) E = E -E = ½ g ħB 0 – (- ½ g ħB 0 ) = g ħB 0 = h L i.e. L = g B 0 / 2 Resonance: The energy of the EM radiation matches the energy gap B 0 = 12T, L = 500 MHz for protons
N uclear M agnetic R esonance Spin ½ nucleus ( 1 H, 13 C) E = E -E = ½ g ħB 0 – (- ½ g ħB 0 ) = g ħB 0 = h L i.e. L = g B 0 / 2 Resonance: The energy of the EM radiation matches the energy gap B 0 = 12T, L = 500 MHz for protons L : precessional frequency
Chemical shift The chemical environment alters the effective magnetic field on the nuclei B eff = B o ( 1 - s ) s = magnetic shielding of the nucleus. Factors that affect it include neighboring atoms, aromatic groups, etc., the polarization of the bonds to the observed nuclei
Chemical shift The chemical environment alters the effective magnetic field on the nuclei B eff = B o ( 1 - s ) s = magnetic shielding of the nucleus. Factors that affect it include neighboring atoms, aromatic groups, etc., the polarization of the bonds to the observed nuclei γB γB - eff 0 ν σ) (1 L 2 π 2 π
Chemical shift The chemical environment alters the effective magnetic field on the nuclei B eff = B o ( 1 - s ) s = magnetic shielding of the nucleus. Factors that affect it include neighboring atoms, aromatic groups, etc., the polarization of the bonds to the observed nuclei γB γB - eff 0 ν σ) (1 L 2 π 2 π 1 H/ 13 C nuclei in different environments resonate at different frequencies
Chemical shift The chemical environment alters the effective magnetic field on the nuclei B eff = B o ( 1 - s ) s = magnetic shielding of the nucleus. Factors that affect it include neighboring atoms, aromatic groups, etc., the polarization of the bonds to the observed nuclei H O-C H 2 -C H 3 γB γB - eff 0 ν σ) (1 L 2 π 2 π low high 1 H/ 13 C nuclei in different environments field L field resonate at different frequencies Intensity Population
The d scale • The frequency of resonance is field-dependent
The d scale • The frequency of resonance is field-dependent • A relative scale, is a less ambiguous representation of the signal of a particular nucleus.
The d scale • The frequency of resonance is field-dependent • A relative scale, is a less ambiguous representation of the signal of a particular nucleus.
The d scale • The frequency of resonance is field-dependent • A relative scale, is a less ambiguous representation of the signal of a particular nucleus. d is field-independent d (-s )
The d scale • The frequency of resonance is field-dependent • A relative scale, is a less ambiguous representation of the signal of a particular nucleus. d is field-independent d (-s ) Reference: Tetramethyl silane ( TMS ) CH3 soluble in most organic solvents, inert, volatile, and has 12 equivalent 1 Hs and 4 equivalent 13 Cs H C CH3 Si 3 Other references: residual solvent peak, dioxane for 13 C, CH3 H 3 PO 4 for 31 P
Characteristic Chemical shifts: 1 H Resonances
Characteristic Chemical shifts: 13 C resonances
Ring currents
Ring currents Shielded
Ring currents Shielded Deshielded
Ring currents Shielded Deshielded
Ring currents Shielded Shielded Deshielded
A representative spectrum: Ethanol
A representative spectrum: Ethanol Three groups of lines = Three kinds of protons
A representative spectrum: Ethanol Three groups of lines = Three kinds of protons Areas: Relative intensities = Abundance
A representative spectrum: Ethanol Three groups of lines = Three kinds of protons Areas: Relative intensities = Abundance What is the significance of the multiplicity of the lines?
The fine structure: Spin-spin Coupling Br CH 3 Small alteration in the magnetic field experienced by a nucleus due to other magnetic nuclei ► Fine structure in the spectra
The fine structure: Spin-spin Coupling Br CH 3 Small alteration in the magnetic field experienced by a nucleus due to other magnetic nuclei ► Fine structure in the spectra
The fine structure: Spin-spin Coupling Br CH 3 Small alteration in the magnetic field experienced by a nucleus due to other magnetic nuclei ► Fine structure in the spectra E = J . I 1 . I 2
The fine structure: Spin-spin Coupling Br CH 3 Small alteration in the magnetic field experienced by a nucleus due to other magnetic nuclei ► Fine structure in the spectra E = J . I 1 . I 2 J Coupling Constant
Recommend
More recommend