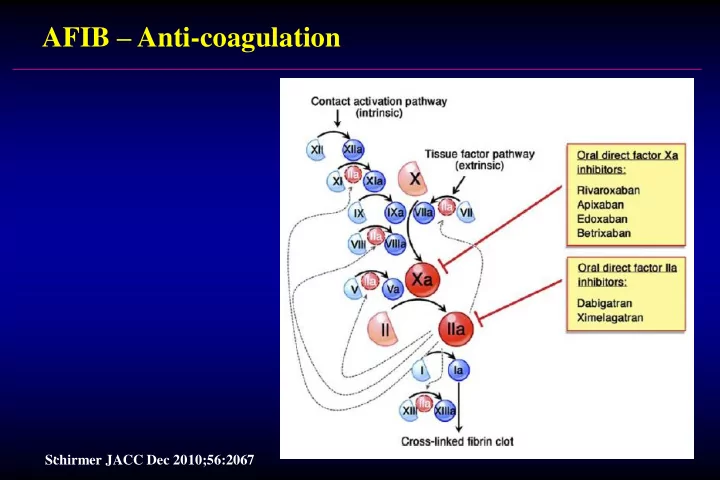

AFIB – Anti-coagulation . Schirmer JACC Dec 2010;56:2067
Clinical Trials - Aristotle • Apixaban 5mg bid vs Warfarin • 18,201 subjects with at least 2 episodes of AFIB and CHADS2 - 1 or more (mean 2.1) • Non-inferiority design • Stroke or systemic embolism as Primary EP • Safety EP was major bleeding • Apixiban previously shown to reduce risk of same EP by 55% in those who could not take Warfarin • Mean age 70 with 2/3 male Grainger et al. NEJM 2011;365;981
Clinical Trials - Aristotle Grainger et al. NEJM 2011;365;981
Clinical Trials - Aristotle Grainger et al. NEJM 2011;365;981
AFIB – ROCKET AFIB • Presented at AHA 2010 • 14000 patients with AFIB randomized to Rivaroxaban or Coumadin • Very high risk with 90% CHADS2 3 or above • 55% had a previous CVA • CVA and systemic embolism over 40 months .
Rivaroxaban vs Warfarin: ROCKET AF Trial 14,264 Patients (Blinded) Per-protocol population Events: stroke or systemic embolism 100 6 HR 0.79 (0.66-0.96) 5 event rate (%) 80 P<0.0001 for noninferiority Cumulative 4 Warfarin 60 3 2 Rivaroxaban 40 1 0 20 0 120 240 360 480 600 720 840 0 0 120 240 360 480 600 720 840 Days since randomization Rates of major bleeding No./100 patient-year Rivaroxaban Warfarin P • Any 2.13 3.09 <0.001 • Fatal 0.4 0.8 0.003 • Intracranial hem 0.33 0.80 <0.001 Patel: NEJM, 2011 With permission B. Gersh 2012 3143509-1
Summary of Recent Clinical Trials Dabigatran Rivaroxaban Apixiban Drug Thrombin Factor Xa Factor Xa Target 150 mgm bid 20 mgm/day 5 mgm bid** Dose 110 mgm bid (not in USA) 75 mgm bid* Endpoints • Stroke/systemic 150 mgm bid – superiority Noninferior Superiority 110 mgm bid – noninferior embolism ( age >75 yrs with 150 • Bleeding mgm bid ) • Intracranial hemorrhage Age >80 yrs *Creatine clearance <30 mL/min **2.5 mgm bid in high-risk pts Wt <60 kg Creatinine >1.5 mgm dL With permission B. Gersh 2012 3169948-4
Clinical Trials – Atlas TIMI 51 • Rivaroxaban 2.5 or 5 mg bid vs placebo • 15,526 ACS patients for mean of 13 months • Primary EP of CV death, MI or stroke • Mean age 61 • 50% STEMI and 60% had PCI or CABG with index event • >90% on ASA and Plavix Mega et al. NEJM 2011;365 (Nov 2011)
Clinical Trials – Atlas TIMI 51 Mega et al. NEJM 2011;365 (Nov 2011)
Clinical Trials – Atlas TIMI 51 Increased IC bleeding 0.6 vs 0.2 % Mega et al. NEJM 2011;365 (Nov 2011)
Clinical Trials – APPRAISE-2 • Higher risk group of ACS patients • HR 0.95 for Apixaban vs placebo for primary EP of death, MI and stroke • At 12 months, event rate almost double ATLAS – ACS2 study Alexander et al. NEJM 2011
Clinical Trials – Stem cell infusion • 8 subjects with endovascular , intra-myocardial injection • Autologous bone marrow derived progenitor cells • Injected into scar and peri-scar areas • CMR end-point at 3, 6 and 12 months • Novel due to looking at chronic scar patients and evaluating reverse remodeling • Preliminary data from TAC- HFT Williams et al. Circ Res 2011;108:792
Clinical Trials – Stem cell infusion • Bone marrow aspirates – Mononuclear cells (n=4) reinfused within 4 hours – Mesenchymal cells (n=4) cultured and reinfused in 5 weeks • Injected by catheter up to 10 areas identified by CMR maps • EF 20-50% and presence of scar on CMR • CMR evaluated EF, volumes, % scar, RWM Williams et al. Circ Res 2011;108:792
Clinical Trials – Stem cell infusion Williams et al. Circ Res 2011;108:792
Clinical Trials – Stem cell infusion Decrease in both ESV and EDV EF doesn’t change Williams et al. Circ Res 2011;108:792
Clinical Trials – Stem cell infusion RWM Decrease in both RWM and amount of scar Early change in RWM predicted later remodeling Williams et al. Circ Res 2011;108:792
Recommend
More recommend