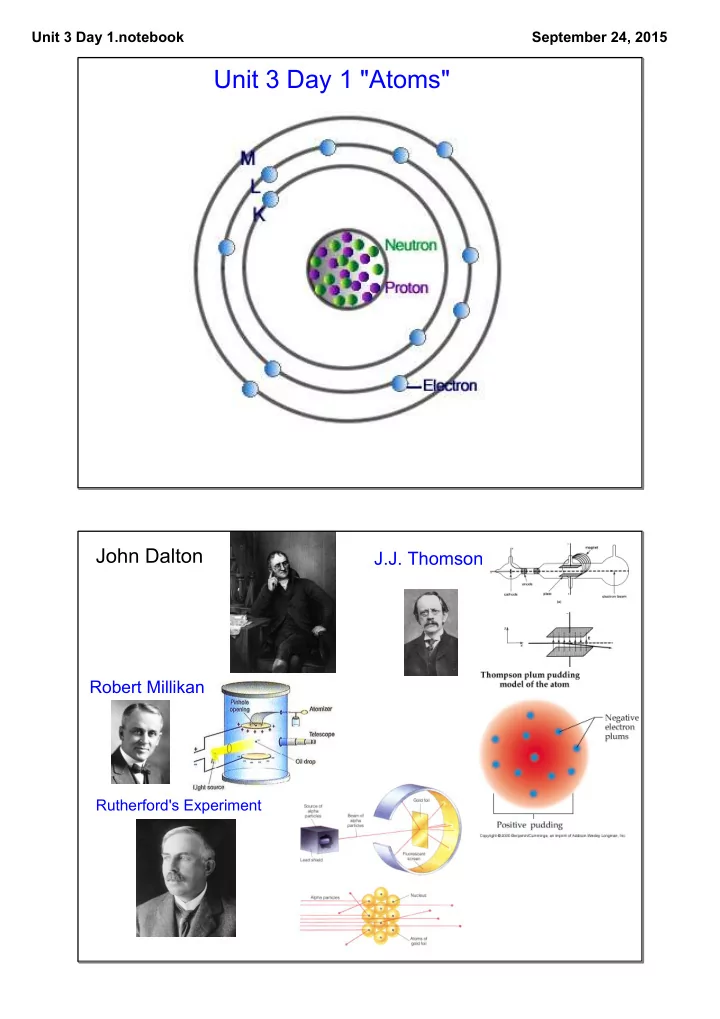

Unit 3 Day 1.notebook September 24, 2015 Unit 3 Day 1 "Atoms" John Dalton J.J. Thomson Robert Millikan Rutherford's Experiment
Unit 3 Day 1.notebook September 24, 2015 Atomic symbols NOT!!! on a periodic table • Mass Number: 1 • # protons + # 81 neutrons Br Charge: only • NOT the average there if it is atomic mass an ion (lost or gained electrons) 35 Atomic Number: symbol • # of protons • if the atom is neutral (not an ion) Said another way: then also the # of e Bromine81 WHAT IT IS!!!!
Unit 3 Day 1.notebook September 24, 2015 Atomic symbols on a periodic table Atomic Number: 35 Br symbol Average atomic mass: 79.91 • average mass of all the isotopes of the element • (mass * %) + (mass*%)...etc 100 Isotope From Wikipedia, the free encyclopedia Isotopes are variants of a particular chemical element. While all isotopes of a given element share the same number of protons, each isotope differs from the others in its number of neutrons Calculating Average Atomic Mass = (27.976927 * 92.23) + (28.976495 * 4.67) + (29.973770 * 3.10) 100
Unit 3 Day 1.notebook September 24, 2015 one of these tells you the other all the same number (if not an ion) (if an ion add or subtract e ) # p + + #n mass# atomic #
Unit 3 Day 1.notebook September 24, 2015 periods group number Name of group: similar properties Alkali earth metals (other names on pg. 124) Alkali metals Alkaline Earth metals Nobel or Inert gases Halogens Transition Metals
Unit 3 Day 1.notebook September 24, 2015 Nuclear Equations Half life calculations Nuclear Equations
Unit 3 Day 1.notebook September 24, 2015 Half life calculations
Attachments Bohratom.swf Rutherford_s Experiment_ Nuclear Atom1.flv Cathode ray tube and electron.flv
Recommend
More recommend